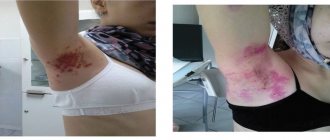
Instructions for use
According to the instructions, Aldara cream is used in the treatment of patients with papillomas (human papillomavirus infection), genital warts (genital or genital warts), molluscum contagiosum, and basal cell carcinoma (basal cell carcinoma). In addition, it is effective for skin regeneration in patients with actinic (senile) keratosis.
Each package of Aldara consists of 12 sachets of 250 mg; Each sachet contains 12.5 mg of imiquimod.
Aldara is used externally. It is applied to the cleaned affected surface and rubbed in with gentle movements until completely absorbed. Since the drug has low absorption through the skin, its overdose is not observed. At the same time, the use of Aldara internally is strictly prohibited.
Aldara should be applied in a thin layer, once a day, three times a week - every other day, preferably at night. Applying cream under a bandage is not recommended.
During the 8-10 hours that the drug is supposed to be on the affected area, you should not take a shower or bath. After this time, Aldara should be washed off with warm water and soap.
Aldara is used until the visible symptoms of the disease disappear, and the course of treatment should not exceed four months.
Introduction
Imiquimod is a synthetic compound that belongs to a new class of drugs called imidazoquinolones. In the body, it acts as an immune response modifier with strong antiviral and antitumor activity. In 1999, the Food and Drug Agency (FDA) approved imiquimod for the treatment of genital and perianal condylomata acuminata, and in 2004 for the treatment of actinic keratoses and superficial basal cell carcinoma. Recently, it has been found that imiquimod may be useful in various dermatological conditions. This article will review the clinical use of Imiquimod 5% cream for precancerous dermatoses and skin malignancies.
Antitumor mechanism of action in malignant skin tumors
Imiquimod acts on the innate and adaptive immune response both directly and indirectly. Its direct action is through binding to Toll-like receptors (TLRs) of macrophages, monocytes and dendritic cells and through the induction of apoptosis. The indirect effect occurs through the induction of immune modulatory cytokines by Imiquimod. The effectiveness of Imiquimod is also due to its action on Langerhans cells, stimulating their ability to present antigens and their migration to the draining lymph nodes, where antigens are presented to T cells, thereby activating the body's adaptive immune response. Recent studies have indicated the possibility of a direct effect of imiquimod on skin malignancies, with the main mechanism in tumor cell lines being the induction of apoptosis. Imiquimod activates apoptosis, namely proteases of the caspase family. There are two pathways that induce apoptosis: extrinsic and intrinsic. Imiquimod has been shown to lead to apoptosis by working through the intrinsic pathway in mitochondria. When the intrinsic apoptosis pathway is activated, pro-apoptotic molecules are released, such as apoptosis-inducing factor, CMAK, HtrA2, cytochrome C and endonuclease G. These molecules then stimulate the activity of caspase-9 in a multimeric complex called the apoptosome or cause the destruction of malignant cells in an independent manner. An independent manner in which Imiquimod induces apoptosis in tumor cell lines is through up-regulation of pro-apoptotic proteins of the Bcl-2 family, namely Bax and Bak, and of the BH3 family only proteins such as BIM, Bid, BMF, NOXA and Puma. Thus, there is up-expression and down-regulation of anti-apoptotic proteins. The indirect action of Imiquimod occurs by inducing the release of various cytokines. These cytokines stimulate the cellular immune response, which may be important for the antitumor activity of the drug. The main induced cytokines are IL-12, tumor necrosis factor alpha (TNF-alpha), interferon (INF)-gamma. They, in turn, increase the levels of cytotoxic T cells and natural killer cells in the local environment by inducing 2'5'oligoadenylate synthetase and block angiogenesis. Further increase in IL-12 downregulates IL-10 and thus stimulates anti-tumor T cells.
Pharmacokinetics
Resorption of 5% Imiquimod cream applied to the skin is minimal. Less than 0.9% of the drug is excreted from the body in urine and feces.
Use of Imiquimod for precancerous and malignant skin tumors
Actinic keratosis
Actinic keratosis is a precancerous skin lesion that is more common in fair-skinned people and is at risk of developing into invasive squamous cell carcinoma. Imiquimod 5% cream was first approved by the FDA as a therapeutic agent for actinic keratoses in 2004 and has shown extremely encouraging results for this pathology. Imiquimod cream has recently been released in a lower concentration of 3.75%, which is as effective as 5% Imiquimod cream, but causes fewer skin side effects. In addition, an additional advantage was the shorter duration of treatment with 3.75% Imiquimod cream and the ability to apply it to a larger area compared to 5% cream (i.e., an area of up to 200 cm 2 compared to 25 cm 2 for the 5% drug Imiquimod).
New mechanisms of action of Imiquimod in actinic keratosis
- In addition to its usual immunomodulatory effect in actinic keratosis, imiquimod stimulates the expression of E-selectin in tumor blood vessels, thereby promoting the activation of CD8+ cytotoxic T cells, leading to tumor regression.
- After using Imiquimod, there is a decrease in the risk of actinic keratosis relapse due to memory T cells remaining in the body.
Imiquimod treatment regimens for actinic keratosis [Table 1]
| Method | Dosage regimen and method of administration | Duration of treatment | Comments |
| Ordinary | Imiquimod 5% cream is applied 3 times a week every other day within the lesions | 8-16 weeks | In most patients, complete regression of the lesions is achieved with histological confirmation of cure. Adverse skin effects may occur |
| Cyclical | Imiquimod 5% cream is applied 3 times a week, every other day, for 4 weeks with a 4-week break. This constitutes one treatment cycle | up to 3 treatment cycles | Achieving clinical remission with more frequent skin side effects compared to conventional treatment |
| Combined with cryotherapy | One cryotherapy session using two 5 second treatments with a 5 second break between treatments. This is followed by the use of 3.75% Imiquimod cream daily for 2 weeks, followed by a 2-week break, after which the drug is applied daily for another 2 weeks. | 6 weeks | Significant improvement in lesions due to the synergistic effect of the combination of these two treatments. Cryotherapy has an immediate cytodestructive effect, which potentiates the slowly developing immunomodulatory effect of Imiquimod. Combination therapy is more effective for hypertrophic actinic keratosis lesions |
| Combined with photodynamic therapy (PTD) | PTD using 20% aminolevulinic acid. 1 treatment per month, after which patients apply 5% Imiquimod cream 2 times a week at 3-day intervals | 16 weeks | This combination therapy is well tolerated by patients. The combination of both treatment methods gives a more pronounced effect on actinic keratosis |
A new, powerful and related drug to Imiquimod is Resiquimod, which is 10-100 times more potent than Imiquimod. A phase II European study demonstrated resolution of 40-74.2% of actinic keratosis lesions when the cream was applied 3 times a week for 4 weeks. Superficial basal cell carcinoma is a common, rarely metastatic skin neoplasm. However, if treatment is neglected, it can proceed aggressively with destruction of the underlying tissues. In addition to the mechanisms of action mentioned above in basal cell carcinoma, Imiquimod has an additional mechanism that consists in blocking the activation of the glioma-associated oncogene (GLI) signaling pathway. It has been suggested that abnormal activation of this pathway plays an important role in the pathogenesis of basal cell carcinoma. Imiquimod binds to adenosine receptors and activates protein kinases, which in turn phosphorylate glioma-associated oncogene, reducing GLI 1 mRNA and protein levels in basal cell carcinoma cells. This has a negative effect on the GLI HH signaling pathway, thereby interfering with its oncogenic potential. The first study using Imiquimod 5% cream for the treatment of superficial basal cell carcinoma was published in 1999. In the study, 35 patients were randomized into several groups with different dosing regimens of 5% cream Imiquimod and a control group using cream base. Treatment was carried out for 16 weeks. If local reactions to the cream occurred, it was discontinued for 7 days. All patients who used the cream 1 to 3 times a day experienced complete resolution of skin rashes, confirmed histologically. When using the cream 2 times a week, complete skin cleansing was observed in 60% of patients. Among patients who applied Imiquimod only once a week, 50% of patients achieved complete remission. The course of treatment in the group applying the cream 2 times a day lasted 10 weeks, in those applying the cream once a day - 13 weeks, and in those applying the cream three times a week - 14.5 weeks.
Key points
- Imiquimod 5% cream was found to be effective in the treatment of small superficial skin tumors up to 2 cm in diameter, as demonstrated by several controlled studies and one systematic review. Imiquimod has also been tested in case series of large superficial basal cell carcinomas larger than 2 cm with good results. A favorable outcome of several cases of basal cell carcinoma after application of 5% Imiquimod was observed in Gorlin syndrome and xeroderma pigmentosum. However, although imiquimod has been used in basal cell carcinomas, it may only be useful as a treatment modality for selected low-risk primary lesions. Surgical techniques such as Mohs micrographic surgery continue to be the gold standard with excellent treatment outcomes.
Nodular basal cell carcinoma
Imiquimod 5% cream can be used as an alternative in patients with small nodular basal cell carcinoma in which surgery is not the first-line treatment. Phase II clinical trials demonstrating the effectiveness of imiquimod in nodular basal cell carcinoma have been completed in a 6-week study in Australia and New Zealand and a 12-week study in the United States. After 6 weeks of treatment, complete, histologically confirmed cure was achieved by 71% of patients who used the cream daily once a day and 76% of patients who used the cream with the same frequency for 12 weeks.
Sclerodermaform basal cell carcinoma
The literature describes a case of successful treatment of sclerodermaform basal cell carcinoma. The use of 5% Imiquimod cream 3 times a week for 16 weeks resulted in complete, histologically confirmed, disappearance of the tumor with the preservation of scar tissue at the site of the tumor. There was no relapse observed over the next 9 months.
Squamous cell carcinoma in situ or Bowen's disease
Squamous cell carcinoma in situ (in situ) can occur on the skin and mucous membranes. Of particular importance is the involvement of the mucous membranes of the genital organs in Bowen's disease. These lesions are usually associated with human papillomavirus, in particular type 16, which is able to suppress the activity of the tumor suppressor proteins p53 and Rb.
New properties of Imiquimod in Bowen's disease with genital localization
When squamous cell carcinoma is localized in the vulvar and anal region, Imiquimod, in addition to its antitumor effects, showed antiviral activity against HPV and, therefore, this action may potentiate the antitumor effect of Imiquimod in this tumor, because Elimination of HPV is a prerequisite for pharmacological therapeutic efficacy.
Main features of the use of Imiquimod for squamous cell carcinoma in situ
| Clinical form of squamous cell carcinoma in situ | Mode of application | Duration of treatment | Comments |
| Skin cancer | Daily application of 5% cream to affected areas | Up to 16 weeks | There were no histological signs of pathology even after 9 months of observation after complete resolution of the tumor |
| Vulvar intraepithelial cancer | Topical application of 5% Imiquimod cream to lesions in the vulvar area, including normal skin adjacent to the tumor, 1 cm from the edge of the tumor, once a week | Until clinical effect is obtained | Pigmented tumors demonstrate better therapeutic results compared to non-pigmented ones. Although surgery is the mainstay of treatment, imiquimod monotherapy may be effective in younger women. Imiquimod may be combined with surgery. |
| Anal intraepithelial cancer | 3 times a week | 16 weeks | Good clinical results are achieved. Regular monitoring is important. Imiquimod therapy can be combined with topical 5-fluorouracil on imiquimod-free days. If there is no effect of therapy within 16 weeks, further therapy makes no sense. |
| Erythroplasia Keira | Topical application of 5% Imiquimod cream 3-7 times a week | 4-24 weeks | Good clinical results are achieved |
Invasive squamous cell carcinoma
Imiquimod 5% cream has been shown to be effective even against invasive squamous cell carcinoma. An additional novel mechanism of action of imiquimod in invasive squamous cell carcinoma is through the antiapoptotic regulator A20, thereby stimulating nuclear transcription factor-Kappa signaling pathways in keratinocytes, ultimately leading to apoptosis and tumor regression. Two kidney transplant patients with invasive squamous cell carcinoma of the skin due to immunodeficiency were treated with Imiquimod 3 times a week for 12 weeks (the first patient) and Imiquimod 3 times a week for 5 weeks and then twice a week for another 7 weeks (the second patient). patient). Follow-up 6 and 8 months (respectively) revealed no evidence of tumor.
Lentigo maligna
Lentigo maligna is an in-situ melanoma. Progression of lentigo maligna to invasive melanoma occurs slowly due to sluggish peripheral growth and is observed in 5 The first report described the successful treatment of lentigo maligna on the scalp in an elderly patient who refused surgical treatment. Treatment was carried out for 7 months and ended in complete clinical and histological remission, without relapses in the subsequent 9 months of observation. In another study, 53-75% of treated patients achieved cure. However, the main limitation for most of these studies was the insufficiently long follow-up period, not exceeding 1 year, which is insufficient in the extremely slow radial growth phase of lentigo maligna.
Basic moments
- Lentigo maligna requires long-term treatment with Imiquimod
- It can be combined with destructive methods such as CO 2 laser
- Even after the lesion has disappeared histologically, it is recommended to continue using Imiquimod for a year.
Metastatic melanoma
Several recent published studies have shown that imiquimod may have promise in the treatment of metastatic melanoma. Imiquimod was first used in a patient with a breast tumor that was too large for surgery or radiation therapy. Imiquimod was applied 3 times a week in combination with systemic dacarbazine. After 12 weeks, lesions treated with Imiquimod were completely resolved. Histological examination after 18 weeks revealed apoptotic melanocytes surrounded by a dense lymphocytic infiltrate.
Recent Postulates on the Role of Imiquimod in the Treatment of Metastatic Melanoma
Recently, the role of imiquimod in suppressing melanoma has been further developed. Imiquimod activates TLR7, stimulating dendritic cells, plasma cells, which accumulate in the tumor and sentinel lymph nodes, where they produce tumor necrosis factor associated with apoptosis-inducing ligands, granzymes, lysozyme, interferon-alpha and other angiogenesis inhibitors. Vascular endothelial growth factor is considered a known angiogenic factor in melanoma, promoting tumor growth. When these substances are released under the influence of Imiquimod, tumor vascularization processes are blocked, and with them tumor growth.
Basic provisions
- Metastatic melanoma requires long-term treatment with imiquimod
- Imiquimod may be used together with other therapies such as radiation therapy and CO 2 laser
- Although imiquimod is able to eliminate cutaneous metastases of melanoma, it is not able to prevent lymphatic spread of the tumor.
Mycosis fungoides
Imiquimod was effective in mycosis fungoides, especially in the treatment of its early forms, which may be due to the ability of Imiquimod to induce the production of interferon-alpha and cytokines.
Key points
- For mycosis fungoides, imiquimod is an alternative treatment for localized plaques that are resistant to conventional treatments.
- Duration of treatment ranges from 4 months to 2 years
- In persistent cases, Imiquimod can be combined with systemic interferons.
Keratoacanthoma
Keratoacanthoma was successfully treated with Imiquimod administered daily for 4 to 12 weeks. Clinical regression was observed after 4-6 weeks. There were no relapses during the 4-6 month follow-up period.
Extramammary Paget's disease
Extramammillary Paget's disease is a clinical condition that histologically mimics Paget's disease of the breast and is clinically characterized by weeping, eczematous and erythematous plaques. The effect of Imiquimod in this pathology is due to its immunomodulatory effect, stimulating the synthesis of proinflammatory cytokines and the induction of apoptosis of malignant cells. In a retrospective study, imiquimod was shown to promote clinical remission at disease onset as well as during relapses. Of the 29 cases of Paget's disease in the vulva, clinical resolution was achieved in 50% of patients with primary disease and in 73% of patients with recurrent disease. In some cases of extramamillary Paget's disease, imiquimod is not able to lead to complete resolution of the lesions. However, even partial reduction in the size of the lesions is beneficial for both the patient and the treating physician because once the lesions are reduced in size, a more gentle surgical approach can be used, resulting in a better cosmetic and functional outcome.
Infantile hemangioma
The effect of Imiquimod here is due to its antiangiogenic properties. A separate study demonstrated the effectiveness of 5% Imiquimod in superficial infantile hemangioma.
Merkel cell carcinoma
Merkel cell carcinoma is a rare neuroendocrine tumor with high malignant potential. We have reported a case of achieving complete remission in Merkel cell carcinoma after using 5% Imiquimod in combination with radiation therapy.
Kaposi's sarcoma
Kaposi's sarcoma is a vascular tumor characterized by reddish-brown nodules and papules. Imiquimod acts by activating cytokines that block angiogenesis, suppress proangiogenic factors, and induce apoptosis in endothelial cells. A successful treatment outcome using Imiquimod 5% cream was reported in an 87-year-old man with extensive Kaposi's sarcoma of both lower extremities. A successful outcome was described in another patient after using 5% Imiquimod cream 3 times a week for localized Kaposi's sarcoma under occlusion for 8 hours after a 3-month course. In localized forms of Kaposi's sarcoma, Imiquimod can be used as monotherapy.
Skin metastases in breast carcinoma
Breast cancer is a frequently diagnosed malignant tumor in women with an increased potential for cutaneous metastasis. The exact mechanism of imiquimod in breast cancer is unknown. However, it has been suggested that by binding to TLR7, imiquimod stimulates dendritic cells and macrophages to release cytokines that accelerate tumor cell apoptosis. Adams and Dr. Devan et al indicated the effectiveness of imiquimod in the treatment of skin metastases in breast cancer. Henriques and further substantiated the positive role of Imiquimod in metastatic breast cancer. Imiquimod was used 3 times a week in combination with chemotherapy, which resulted in a decrease in lesion thickness and pain.
Side effects
The most common side effects of Imiquimod are itching, burning, pain and tenderness at the application site. Other local reactions include erythema, erosions, desquamation, crusting and vesicles. Less common side effects include hypopigmentation, hyperkeratosis, rhinitis, upper respiratory tract infections, myalgia and headache. Fatigue, dyspepsia, alopecia, chills, diarrhea, fever and angioedema have been rarely reported.
conclusions
Imiquimod 5% cream has demonstrated effectiveness in a variety of skin cancer conditions. Unfortunately, the effectiveness of imiquimod has primarily been studied in the form of case reports or small open-label studies. There are very few double-blind crossover studies. There is a need for larger, multicenter studies that would better assist clinicians in successfully utilizing the immune modifier activity of Imiquimod in a variety of dermatological conditions.