Compound
One tablet of the drug may contain 2.5 / 5 / 10 / 20 / 50 / 100 or 200 mg of torasemide as the active ingredient.
Additional ingredients of tablets of 2.5 / 5 / 10 and 20 mg: corn starch, lactose, colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose.
Additional ingredients: 50 tablets; 100 and 200 mg: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, mannitol (E 421), crospovidone, copolyvidone, hydrogenated castor oil.
Pharmacodynamics and pharmacokinetics
Torsemide Sandoz belongs to a group of diuretics called loop diuretics . Small doses of torasemide are used to treat arterial hypertension , both in monotherapy and in combination with other antihypertensive drugs , where torasemide exhibits a mild saluretic and diuretic effect. Higher doses of the drug have enhanced diuretic effects depending on the dose used. The maximum diuretic activity of Torasemide Sandoz is observed 2-3 hours after oral (internal) administration.
Internal administration of torasemide leads to rapid absorption and almost complete absorption. Plasma Cmax is observed after 60-120 minutes, systemic bioavailability is 80-90%. Connection with plasma proteins is more than 99%, with a volume of distribution equal to 16 liters.
Metabolism occurs by hydroxylation and oxidation with the release of 3 metabolites: M1, M3 and M5, where M1 and M3 are active (about 10% of the pharmacological effects of the drug).
In healthy people, the final half-life of torasemide , as well as its metabolites, is 3-4 hours. Renal clearance is approximately 10 ml/min with a total clearance of 40 ml/min.
Approximately 80% of the dose is excreted, the unchanged torasemide (24%) and its metabolic products M1 (12%), M3 (3%) and M5 (41%).
In case of renal failure, T½ of the drug metabolites (M3 and M5) increases, although T½ of torasemide remains unchanged. Hemofiltration and hemodialysis are practically ineffective.
In case of heart failure and liver pathologies, T½ of the M5 metabolite and the active ingredient is slightly prolonged, but without proven cumulation of the drug.
Torasemid-SZ
Registration number
LP-003307
Dosage form
pills
Compound
1 tablet contains:
dosage 5 mg:
active substance: torasemide - 5 mg excipients: lactose monohydrate (lacto press) (milk sugar) - 89.3 mg; pregelatinized starch (starch 1500) - 24.5 mg; colloidal silicon dioxide (aerosil) - 0.6 mg; magnesium stearate - 0.6 mg.
dosage 10 mg:
active substance: torasemide - 10 mg excipients: lactose monohydrate (lacto press) (milk sugar) - 178.6 mg; pregelatinized starch (starch 1500) - 49.0 mg; colloidal silicon dioxide (aerosil) -1.2 mg; magnesium stearate - 1.2 mg.
Description
Tablets are white or almost white, round, flat cylindrical with a chamfer and a score on one side.
ATX code
C03CA04
Pharmacological properties
Pharmacodynamics
Torsemide is a loop diuretic. The maximum diuretic effect develops 2-3 hours after taking the drug orally. The main mechanism of action of the drug is due to the reversible binding of torasemide to the sodium/chlorine/potassium ion contransporter located in the apical membrane of the thick segment of the ascending loop of Henle, as a result of which the reabsorption of sodium ions is reduced or completely inhibited and the osmotic pressure of intracellular fluid and water reabsorption are reduced. . Blocks myocardial aldosterone receptors; reduces fibrosis and improves diastolic myocardial function.
Torasemide causes hypokalemia to a lesser extent than furosemide, but it is more active and its action lasts longer. The use of torasemide is the most reasonable choice for long-term therapy.
Pharmacokinetics
After oral administration, torasemide is quickly and almost completely absorbed from the gastrointestinal tract. Food intake does not have a significant effect on the absorption of the drug. The maximum concentration of torasemide in the blood plasma is observed 1-2 hours after oral administration. Bioavailability is 80 - 90% with minor individual variations.
The diuretic effect lasts up to 18 hours, which facilitates the tolerability of therapy due to the absence of very frequent urination in the first hours after taking the drug orally, which limits the activity of patients.
Communication with blood plasma proteins is more than 99%. The apparent volume of distribution is 16 l. Metabolized in the liver using isoenzymes of the cytochrome P450 system. As a result of sequential reactions of oxidation, hydroxylation or ring hydroxylation, three metabolites are formed (M1, M3 and M5), which bind to plasma proteins by 86%, 95% and 97%, respectively.
The half-life (T1/2) of torasemide and its metabolites is 3-4 hours and does not change in chronic renal failure. The total clearance of torasemide is 40 ml/min, renal clearance is 10 ml/min. On average, about 83% of the dose taken is excreted by the kidneys: unchanged (24%) and in the form of predominantly inactive metabolites (M1 - 12%, M3 - 3%, M5 -41%). In renal failure, T1/2 does not change, T1/2 of metabolites M3 and M5 increases. Torsemide and its metabolites are slightly eliminated by hemodialysis and hemofiltration.
In case of liver failure, the concentration of torasemide in the blood plasma increases due to a decrease in the metabolism of the drug in the liver. In patients with cardiac or liver failure, T1/2 of torasemide and metabolite M5 is slightly increased, drug accumulation is unlikely.
Indications for use
• edema syndrome of various origins, including in chronic heart failure, diseases of the liver, lungs and kidneys;
• arterial hypertension.
Contraindications
Hypersensitivity to torsemide or to any of the components of the drug; in patients with an allergy to sulfonamides (sulfonamide antimicrobials or sulfonylureas); renal failure with anuria; hepatic coma and precoma; refractory hypokalemia/refractory hyponatremia; hypovolemia (with or without arterial hypotension) or dehydration; pronounced disturbances in the outflow of urine of any etiology (including unilateral damage to the urinary tract); glycoside intoxication; acute glomerulonephritis; decompensated aortic and mitral stenosis, hypertrophic obstructive cardiomyopathy; increased central venous pressure (over 10 mm Hg); hyperuricemia; simultaneous use of aminoglycosides and cephalosporins; age under 18 years; pregnancy, breastfeeding period; lactose intolerance, lactase deficiency or glucose-galactose malabsorption.
CAREFULLY
Arterial hypotension, stenosing cerebral artery atherosclerosis, hypoproteinemia, predisposition to hyperuricemia, urinary flow disorders (benign prostatic hyperplasia, narrowing of the urethra or hydronephrosis), history of ventricular arrhythmia, acute myocardial infarction (increased risk of cardiogenic shock), diarrhea , pancreatitis, diabetes mellitus (decreased glucose tolerance), hepatorenal syndrome, gout, anemia; simultaneous use of cardiac glycosides, corticosteroids and adrenocorticotropic hormone (ACTH).
USE IN PREGNANCY AND DURING THE PERIOD
Torsemide does not have a teratogenic effect or fetotoxicity; it penetrates the placental barrier, causing disturbances in water-electrolyte metabolism and thrombocytopenia in the fetus.
There have been no controlled studies on the use of torasemide in pregnant women; the drug is not recommended for use during pregnancy. It is not known whether torasemide passes into breast milk. If it is necessary to use the drug Torasemide-SZ during lactation, you must stop breastfeeding.
Directions for use and doses
Orally, once a day, without chewing, with a sufficient amount of water. The tablets can be taken at any convenient time, regardless of meals. Edema syndrome in chronic heart failure
The usual starting dose is 10-20 mg once daily. If necessary, the dose can be doubled until the desired effect is obtained. Edema syndrome in kidney disease
The usual starting dose is 20 mg once daily. If necessary, the dose can be doubled until the desired effect is obtained. Edema syndrome in liver disease
The usual starting dose is 5-10 mg once daily. If necessary, the dose can be doubled until the desired effect is obtained. The maximum single dose is 40 mg; it is not recommended to exceed it (there is no experience with use). The drug is used for a long period or until swelling disappears.
Arterial hypertension
The starting dose is 2.5 mg (1/2 5 mg tablet) once daily. If there is no therapeutic effect within 4 weeks, the dose is increased to 5 mg once a day. If there is no adequate reduction in blood pressure when taken at a dose of 5 mg once a day for 4-6 weeks, the dose is increased to 10 mg once a day. If the use of the drug at a dose of 10 mg per day does not give the required effect, an antihypertensive drug of another group is added to the treatment regimen.
Elderly patients do not require dose adjustment.
Side effect
The incidence of side effects is classified according to the recommendations of the World Health Organization: very common: > 1/10 (> 10%); often: from>1/100 to <1/10 (>1% and <10%);
uncommon: > 1/1000 to < 1/100 (> 0.1% and < 1%); rare: from > 1/10000 to < 1/1000 (> 0.01% and < 0.1%); very rare: <1/10000 (<0.01%); frequency unknown: frequency cannot be estimated from available data. From the nervous system: often - headache, dizziness, drowsiness; infrequently - muscle cramps of the lower extremities; frequency unknown - confusion, fainting, parasthesia in the extremities (feeling of numbness, “crawling” and tingling). From the senses: frequency unknown - visual impairment, hearing impairment, tinnitus and hearing loss (usually reversible), usually in patients with renal failure or hypoproteinemia (nephrotic syndrome). From the cardiovascular system: infrequently - extrasystole, arrhythmia, tachycardia; frequency unknown - excessive decrease in blood pressure, orthostatic hypotension, collapse, deep vein thrombosis, thromboembolism, decrease in circulating blood volume. From the respiratory system: infrequently - nosebleeds.
From the digestive system.
often - diarrhea; uncommon - abdominal pain, flatulence, polydipsia; frequency unknown - dry mouth, nausea, vomiting, loss of appetite, pancreatitis, dyspeptic disorders, intrahepatic cholestasis. From the skin and subcutaneous tissues: frequency unknown - skin itching, rash, urticaria, erythema polymorphosus, exfoliative dermatitis, purpura, vasculitis, photosensitivity. From the musculoskeletal system: frequency unknown - muscle weakness. From the urinary system: often - increased frequency of urination, polyuria, nocturia; infrequently - frequent urge to urinate; frequency unknown - oliguria, urinary retention (in patients with urinary tract obstruction), interstitial nephritis, hematuria. From the reproductive system: frequency unknown - decreased potency. From the metabolic side: frequency unknown - hypokalemia, hyponatremia, hypomagnesemia, hypocalcemia, hypochloremia, metabolic alkalosis, hypovolemia, dehydration (more often in elderly patients). From laboratory parameters: infrequently - hypercholesterolemia, hypertriglyceridemia; frequency unknown - hyperuricemia, a slight increase in the activity of alkaline phosphatase in the blood plasma, an increase in the concentration of creatinine and urea in the blood plasma, an increase in the activity of some “liver” enzymes in the blood plasma (for example, gamma-glutamyl transferase), thrombocytopenia, leukopenia, agranulocysis toz, hyperglycemia, decreased glucose tolerance (possible manifestation of latent diabetes mellitus).
Other:
frequency unknown - aplastic or hemolytic anemia.
Overdose
Symptoms: increased diuresis, accompanied by a decrease in circulating blood volume and disturbances in the water-electrolyte balance of the blood, followed by a pronounced decrease in blood pressure, drowsiness and confusion, and collapse. Gastrointestinal disturbances may occur.
Treatment: there is no specific antidote. Provocation of vomiting, gastric lavage, activated charcoal. Treatment is symptomatic, dose reduction or discontinuation of the drug and at the same time replenishment of blood volume and indicators of water-electrolyte balance and acid-base status under the control of serum concentrations of electrolytes, hematocrit, symptomatic treatment.
Hemodialysis is ineffective.
Interaction with other drugs
Increases the concentration and risk of developing nephro- and ototoxic effects of cephalosporins, aminoglycosides, chloramphenicol, ethacrynic acid, cisplatin, amphotericin B (due to competitive renal excretion). Increases the effectiveness of diazoxide and theophylline, reduces the effectiveness of hypoglycemic agents, allopurinol. Pressor amines and torasemide mutually reduce the effectiveness. Drugs that block tubular secretion increase the concentration of torasemide in the blood serum. With the simultaneous use of glucocorticosteroids, amphotericin B, the risk of developing hypokalemia increases, with cardiac glycosides - the risk of developing glycoside intoxication increases due to hypokalemia (for high- and low-polarity) and prolongation of the half-life (for low-polarity). Reduces the renal clearance of lithium drugs and increases the likelihood of intoxication. Nonsteroidal anti-inflammatory drugs, sucralfate, reduce the diuretic effect due to inhibition of prostaglandin synthesis, disruption of renin activity in the blood plasma and excretion of aldosterone. Strengthens the antihypertensive effect of antihypertensive drugs, neuromuscular blockade of depolarizing muscle relaxants (suxamethonium) and weakens the effect of non-depolarizing muscle relaxants (tubocurarine). Concomitant use of large doses of salicylates during torsemide therapy increases the risk of their toxicity (due to competitive renal excretion). Sequential or simultaneous use of Torsemide with angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor antagonists can lead to a significant decrease in blood pressure. This can be avoided by reducing the dose of torasemide or temporarily stopping it.
Concomitant use of probenecid or methotrexate may reduce the effectiveness of torsemide (same secretion route). On the other hand, torasemide may lead to decreased renal elimination of these drugs.
With simultaneous use of cyclosporine and torasemide, the risk of developing gouty arthritis increases due to the fact that cyclosporine can cause impaired renal urate excretion, and torasemide can cause hyperuricemia.
It has been reported that in patients at high risk of developing nephropathy taking torasemide orally, renal dysfunction was observed more often during the administration of radiocontrast agents than in patients at high risk of developing nephropathy who received intravenous hydration before the administration of radiocontrast agents. The bioavailability and, as a consequence, the effectiveness of torasemi may be reduced when combined with cholestyramine.
special instructions
Use strictly as prescribed by your doctor. Patients with hypersensitivity to sulfanilamides and sulfonylurea derivatives may have cross-sensitivity to Torasemide-SZ. For patients receiving high doses of Torasemide-SZ for a long period, in order to avoid the development of hyponatremia, metabolic alkalosis and hypokalemia, a diet with sufficient salt content and the use of potassium supplements are recommended.
An increased risk of developing fluid and electrolyte imbalances is observed in patients with renal failure. During the course of treatment, it is necessary to periodically monitor the concentration of blood plasma electrolytes (including sodium, calcium, potassium, magnesium), acid-base status, residual nitrogen, creatinine, uric acid and, if necessary, carry out appropriate corrective therapy (with a higher frequency in patients with frequent vomiting and against the background of parenterally administered fluids).
If azotemia and oliguria appear or worsen in patients with severe progressive kidney disease, it is recommended to suspend treatment.
The selection of a dosage regimen for patients with ascites against the background of liver cirrhosis should be carried out in hospital conditions (violations of water and electrolyte balance can lead to the development of hepatic coma). Regular monitoring of blood plasma electrolytes is indicated for this category of patients.
In patients with diabetes mellitus or with reduced glucose tolerance, periodic monitoring of glucose concentrations in the blood and urine is required. In unconscious patients with prostatic hyperplasia and narrowing of the ureters, diuresis control is necessary due to the possibility of acute urinary retention.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, patients should refrain from driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions (risk of dizziness and drowsiness).
Release form
Tablets 5 mg and 10 mg. 10 tablets per blister pack. 30 tablets in a polymer jar made of low-density polyethylene with a lid made of high-density polyethylene or in a polymer bottle made of low-density polyethylene with a lid made of high-density polyethylene. Each jar, bottle, 2, 3, 6, 10 blister packs along with instructions for use are placed in a cardboard box.
Best before date
3 years. Do not use after the expiration date stated on the packaging.
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 ° C. Keep out of the reach of children.
Vacation conditions
Dispensed by prescription.
Prescription drug
Prescription drug
Indications for use
- For tablets with a mass content of torasemide 2.5 and 5 mg: – therapy of essential hypertension .
- For tablets with a mass content of torasemide 5; 10 and 20 mg: – prevention or reduction of edema in patients suffering from heart failure .
- For tablets with a mass content of torasemide 50; 100 and 200 mg: - prevention or reduction of edema , as well as increased blood pressure in renal failure (CRF) with CC less than 20 ml/min, provided there is a residual diuresis of more than 200 ml per day, including patients on hemodialysis .
Contraindications
- high sensitivity to torasemide , or additional ingredients of the drug;
- hepatic precoma or coma ;
- kidney failure with anuria ;
- arrhythmia;
- arterial hypotension;
- hyponatremia;
- age under 18 years;
- hypovolemia;
- periods of breastfeeding and pregnancy ;
- hypokalemia;
- serious problems during urination , for example due to prostate adenoma .
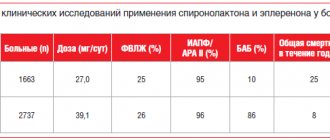
Edema-ascitic syndrome is the most common complication of liver cirrhosis. Its appearance indicates progression of the disease and is associated with a 50% mortality rate over the next 5 years [1–3]. The key drugs for the treatment of edematous-ascitic syndrome include loop diuretics, which improve the quality of life of patients and prevent the development of spontaneous bacterial peritonitis and hepatorenal syndrome. Conservative treatment includes an aldosterone antagonist, spironolactone, and loop diuretics—furosemide and torsemide [4–6]. This combination makes it possible to achieve regression of edematous-ascitic syndrome in approximately 60–75% of patients, especially in the early stages of treatment. For some patients, diuretic therapy may be ineffective due to loss of sensitivity to diuretic therapy with the formation of refractory ascites, in the presence of which the one-year survival rate is only 25% [7]. The use of diuretics in recommended doses limits such adverse events as hypotension, electrolyte disturbances, deterioration of renal function and azotemia, and an increase in hepatic encephalopathy [1, 4]. Resistance to diuretics and side effects of the traditional regimen provided the impetus for the search for new treatment algorithms for patients with edematous-ascitic syndrome. As an alternative, torasemide SR has proven itself, the clinical advantages of which include a greater diuretic effect and a lower risk of developing electrolyte disturbances [1, 8–10]. Important features of the drug (unlike furosemide) are its blocking effect on aldosterone receptors and the absence of the rebound phenomenon, which is associated with sodium and water retention. This makes the use of torasemide SR justified in liver cirrhosis. Torsemide has proven its effectiveness in both patients with chronic heart failure [11–13] and patients with liver cirrhosis. Compared with furosemide, therapy with torasemide SR was accompanied by a decrease in the number of hospitalizations and a decrease in mortality in patients with edematous-ascitic syndrome in cirrhosis of the liver [14]. In recent years, sustained-release torsemide (SR) has become available. Possessing all the properties of torasemide, the prolonged form (Britomar) provides a gradual release of the drug, which allows reducing fluctuations in the concentration of torasemide in the blood plasma [15, 16]. High and predictable bioavailability and a long half-life determine the stability of natriuresis and diuretic effect of torasemide 3V, minimize electrolyte disturbances, and improve the quality of life of patients [17–19]. The use of torasemide ZV in patients with edematous-ascitic syndrome against the background of liver cirrhosis has not been sufficiently studied.
The purpose of the study was to evaluate the effectiveness and safety of the use of torasemide ZV (Britomar) in patients with edematous-ascitic syndrome against the background of decompensated liver cirrhosis.
Material and methods
The study included 42 patients (including 20 women) with liver cirrhosis and edematous-ascitic syndrome. The average age was 57.4±11.5 years. Exclusion criteria were age <18 years, hepatorenal syndrome, hyponatremia <125 mmol/L, hypokalemia <3.5 mmol/L, hyperkalemia >5.5 mmol/L, worsening hepatic encephalopathy or hepatic coma, hypersensitivity to extended-release torsemide , anuria, sinoatrial and atrioventricular blockade of II–III degrees, arterial hypotension (BP <90/60 mm Hg). The study did not include patients with severe acute alcoholic hepatitis.
All patients followed a diet limiting table salt to 3 g/day, abstained from drinking alcohol and received standard therapy for liver cirrhosis: transfusion of albumin, fresh frozen plasma, lactulose, ciprofloxacin, propranolol.
During randomization, patients were divided into 2 groups. The main group received torasemide ZV (n=20), the control group received furosemide (n=22). All patients received spironolactone. The drugs were taken orally in tablet form for an average of 3 weeks. The initial doses of diuretics (torasemide ZV 10 mg, furosemide 40 mg, spironolactone 100 mg/day) were increased by 2 times every 3 days in case of body weight loss of less than 300 g/day. The limitation was a decrease in body weight of more than 500 g/day in patients without peripheral edema [1, 5, 6].
All patients underwent a clinical assessment, laboratory examination, which included a complete blood count, biochemical parameters of blood serum (total protein, albumin, creatinine, electrolytes, alkaline phosphatase, bilirubin, alanine aminotransferase - ALT, aspartate aminotransferase - AST, γ-glutamyl transpeptidase, coagulation system parameters, general urine analysis, daily urinary sodium excretion. In addition, instrumental studies were carried out, including electrocardiography, chest radiography, ultrasound examination of the liver, esophagogastroduodenoscopy. Ultrasound elastometry of the liver using the Fibroscan 502 Touch device (Echosens, France) was performed in all patients after reduction of ascites. The alcoholic genesis of liver cirrhosis was confirmed by the presence of signs of chronic alcohol intoxication. All patients underwent a serological study of markers of viral hepatitis. In patients under 35 years of age, ceruloplasmin was studied. To exclude hemochromatosis, serum iron and ferritin were studied.
The effectiveness and safety of therapy was assessed daily using the following indicators: daily diuresis, body weight, abdominal circumference, edema, blood pressure (BP), heart rate. The level of electrolytes and creatinine in the blood was determined every 3 days, and daily natriuresis was determined before and after diuretic therapy.
Statistical processing of the obtained data was carried out using the Statistica 7.0 program. To assess the statistical significance of differences between groups, nonparametric Mann–Whitney and Wilcoxon tests were used. Correlation analysis was carried out using Spearman statistics. All data are presented as means ± SD. Differences were considered statistically significant at p<0.05.
results
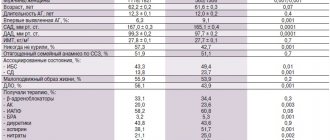
According to the main clinical and demographic indicators, the study groups were comparable (Table 1). In all patients, liver cirrhosis was of alcoholic origin; in 31%, markers of viral hepatitis were detected; in 71%, liver cirrhosis corresponded to Child–Pugh class C. In 14.2% of them, the diagnosis of liver cirrhosis was established for the first time. In all patients, the course of liver cirrhosis was complicated by the development of ascites; in 86%, ascites had grade III severity; 43% had previously taken diuretic therapy. No patient required laparocentesis either earlier or during the study. All of them had swelling of the lower extremities, jaundice, signs of hepatic encephalopathy, and a decrease in the synthetic function of the liver.
The liver density in the torasemide SV group averaged 56±8 kPa, in the furosemide group – 60±10 kPa. By the end of the study, the average dose of torasemide pollutants was 8±2 mg/day, furosemide 60.0±35 mg/day. At the same time, in the furosemide group, the dose of furosemide was increased in 11 (50%) patients, and 2 times in 7. In the torasemide 3 group, no dose increase was required. The groups were comparable in terms of concomitant therapy. Against the background of diuretic therapy, a decrease in edema syndrome was noted in both groups: in the torasemide ZV group, 2 (10%) people remained pasty in the legs and feet; in the furosemide group – in 6 (27%).
All patients experienced regression of ascites to grade I. A more pronounced diuretic effect was observed in the torasemide ZV group.
In the torasemide group, diuresis increased on average by 865±660 ml, and in the furosemide group – by 400±300 ml (p=0.018). At the same time, no statistically significant differences in the dynamics of body weight were detected between the groups: in the torasemide ZV group, body weight decreased by 8±4 kg, in the furosemide group - by 5.8±3.8 kg (p=0.06).
In the long-acting torsemide group, there was a statistically significant increase in daily urinary sodium excretion (93±63.0 mmol/day compared to the furosemide group 51±20 mmol/day; p=0.012). There were no statistically significant differences in the levels of potassium and sodium in the blood plasma in the two groups.
During treatment, renal function was stable, creatinine levels did not increase.
The effect of diuretic therapy on blood pressure, electrolytes and creatinine levels is presented in Table. 2. By the end of the study, all patients showed a statistically significant decrease in the average Child–Pugh score (p = 0.003) and a decrease in the severity of liver cirrhosis (p = 0.003). However, no statistically significant differences were found between the groups (Figure).
Thus, when comparing torasemide ZV with furosemide in the study group, a significantly greater diuretic and saluretic effect was obtained. At the same time, no statistically significant differences in body weight loss and regression of edema syndrome were identified.
Discussion
For the treatment of edematous-ascitic syndrome in patients with decompensated liver cirrhosis, immediate-release torasemide has been most studied. A number of studies have demonstrated that the pharmacokinetics of torasemide IV, in particular bioavailability, are comparable to those of immediate-release torasemide [20]. In liver cirrhosis, an increase in bioavailability (2.5 times) and half-life of torsemide (up to 4.8 hours) was noted [21].
However, an increase in accumulation with long-term use of the drug is not expected, because in such patients, about 80% of the drug dose was excreted in the urine per day (unchanged and in the form of metabolites), i.e. no dose adjustment is required for liver cirrhosis [22].
In the treatment of edematous-ascitic syndrome in patients with liver cirrhosis, it is possible to use torasemide both as monotherapy and in combination with spironolactone [23]. Torsemide therapy is indicated both for decompensation of ascites and for its prevention. Torsemide does not cause hypokalemia even with long-term use [24].
To evaluate the effectiveness of diuretic therapy in patients with liver cirrhosis and ascites, a number of comparative studies of torasemide and furosemide were conducted. A randomized study of 28 patients with ascites treated with spironolactone (200 mg/day) compared the results of 6 weeks of therapy with torasemide (20 mg/day) and furosemide (40 mg/day). Both drugs had comparable effects on body weight, diuresis, and excretion of uric acid, sodium, and chloride, but excretion of potassium, calcium, inorganic phosphate, and magnesium was lower in the torsemide group [25].
In a double-blind crossover study, the results of oral administration of furosemide (80 mg) and torasemide (20 mg) were compared in 14 patients. Torsemide was superior to furosemide in diuretic and natriuretic activity.
Five patients had a weak response to furosemide, while torasemide caused a significant increase in natriuresis and diuresis [26].
The positive effect of torasemide on diuresis and saluresis was shown in a study involving 124 patients with liver cirrhosis: 61 people received torasemide and 63 received furosemide. Doses were selected individually, taking into account the gradation of ascites and response to diuretic therapy. In the torsemide group, daily urinary sodium excretion significantly increased compared with that in the furosemide group (p < 0.05) [10].
An increase in natriuresis (p=0.012) and diuresis (p=0.018) was also observed in our study when using torasemide ZV. A group of 46 patients with liver cirrhosis complicated by ascites (randomized trial) were treated with torasemide 20 mg/day or furosemide 40 mg/day in combination with spironolactone 200 mg/day. If it was not possible to achieve a weight loss of 300 g/day, the doses of diuretics were increased every 3 days to 40, 120 and 400 mg/day, respectively. Torasemide ZV caused a more pronounced increase in diuresis than furosemide, although in general the treatment results in the 2 groups were comparable. Increases in diuretic doses were required in 2 patients in the torasemide 3V group and in 9 patients in the furosemide group (p<0.05) [15].
In our study, when comparing torasemide ZV with furosemide, no statistically significant differences in the dynamics of body weight were detected (p = 0.06). The lack of significant differences between the average body weight of the experimental group and the comparison group may be due to the targeted dosed weight reduction in both groups according to the recommended regimens. It is worth noting that an increase in diuretic doses was required only in the furosemide group.
Torsemide did not affect plasma blood pressure, creatinine and electrolyte levels when using the drug at a dose of 20 mg/day compared with furosemide at a dose of 50 mg/day [22, 27]. The duration of diuretic therapy was 7 days. In our study, patients took torasemide ZV for an average of 3 weeks. In the study group, there was a trend towards lower blood pressure levels before inclusion in the study. However, Britomar had no effect on blood pressure levels. There were no changes in the levels of potassium, sodium and creatinine in the blood.
One study showed that during therapy with torasemide, compared with furosemide, treatment-resistant ascites develops significantly less often (16.4 and 38.1%; p<0.05). The duration of therapy was 15 days [9]. However, only patients with grade III ascites were included. More than 50% of patients had recurrent ascites that could not be corrected with diuretic therapy on an outpatient basis. A decrease in the synthetic function of the liver (hypoalbuminemia) was corrected by transfusion of fresh frozen plasma only. In our study, loss of sensitivity to diuretics was not detected in any of the groups. This may be due to concomitant therapy. Thus, the main method of correcting hypoproteinemia was the transfusion of albumin, rather than fresh frozen plasma. In addition, the severity of ascites was less pronounced. The study involved patients with stage II ascites, 42% had not previously received diuretic therapy, and in 14.2% liver cirrhosis debuted with edematous-ascitic syndrome.
When planning the study, we assumed that the use of torasemide ZV would reduce the length of hospital stay due to a more rapid regression of the edematous-ascitic syndrome. There were no significant differences in the length of stay of patients in the hospital. The dose ratio of torasemide and furosemide is 10–20 and 40 mg/day [21, 28]. In most studies, the starting dose of immediate-release torasemide was 20 mg/day [9, 23–27]. In our study, approximately 50% of patients had not previously received diuretic therapy, had relatively preserved natriuresis and a trend towards lower blood pressure. In this regard, the initial dose of torasemide pollutant was 10 mg/day. During the therapy, positive dynamics were observed in the form of increased diuresis and natriuresis without the development of hypotension, electrolyte disturbances, and a stable creatinine level.
Given the calm safety profile of torasemide ZV, it is likely that a more rapid clinical effect will be observed with increasing doses.
Thus, torasemide ZV (Britomar) can serve as an alternative to furosemide in the treatment of edematous-ascitic syndrome in patients with decompensated liver cirrhosis.
Side effects
Hepatobiliary system:
- an increase in the blood plasma content of certain liver enzymes , in particular γ-glutamyl transpeptidase.
The cardiovascular system:
- cerebral and cardiac ischemia with the risk of developing heart rhythm , angina pectoris , myocardial infarction ;
- arterial hypotension;
- thrombosis;
- fainting states.
Blood system and lymphatic system:
- a decrease in the number of platelets , leukocytes , erythrocytes , or a combination of them.
Gastrointestinal organs:
- loss of appetite;
- stomach pain;
- nausea;
- flatulence;
- vomit;
- pancreatitis;
- diarrhea or constipation .
Skin:
- various allergic manifestations , such as exanthema , itching , photosensitivity , rash (there is evidence of the development of severe skin reactions).
Urinary system:
- an increase in plasma levels of urea and creatinine ;
- urge to urinate;
- urinary disorders , for example with prostate adenoma , bladder expansion and urinary retention .
Metabolism:
- metabolic alkalosis;
- spasms of muscle tissue (especially at the beginning of therapy);
- hypokalemia in patients with chronic liver dysfunction, with diarrhea or vomiting , concomitant low-potassium diet , excessive use of laxatives;
- an increase in the blood plasma content of glucose , uric acid and lipids (cholesterol, triglycerides);
- disturbances of electrolyte and water balance of varying severity, depending on the duration of therapy and dosage of the drug;
- arterial hypotension , asthenia , headaches , drowsiness due to increased urination, which leads to significant fluid loss, especially at the beginning of therapy and in elderly patients.
Are common:
- general weakness (especially at the beginning of therapy);
- headache;
- dry mouth;
- confusion;
- visual disturbances;
- dizziness;
- noise in ears;
- increased fatigue;
- hearing loss;
- paresthesia of the limbs.
Torasemide
The frequency of the side effects listed below was determined according to the following (World Health Organization classification): very common (more than 10%); often (more than 1% and less than 10%); uncommon (more than 0.1% and less than 1%); rare (more than 0.01% and less than 0.1%); very rarely (less than 0.01%), including isolated reports; frequency unknown (cannot be estimated from available data).
From the blood and lymphatic system
Frequency not known - thrombocytopenia, leukopenia, agranulocytosis, aplastic or hemolytic anemia.
Metabolism and nutrition
Uncommon: hypercholesterolemia, hypertriglyceridemia.
The frequency is unknown - decreased glucose tolerance (possible manifestation of latent diabetes mellitus).
From the nervous system
Often - dizziness, headache, drowsiness; infrequently - muscle cramps of the lower extremities; frequency is unknown - confusion, fainting, paresthesia in the extremities (feeling of numbness, “crawling” and tingling).
From the side of the organ of vision
Frequency unknown - visual impairment.
Hearing and labyrinth disorders
Frequency not known? 1a - hearing impairment, tinnitus and hearing loss (usually reversible) usually in patients with renal failure or hypoproteinemia (nephrotic syndrome).
From the cardiovascular system
Uncommon: extrasystole, tachycardia, increased heart rate, facial flushing; frequency not known - excessive arterial hypotension, orthostatic hypotension, collapse, deep vein thrombosis, thromboembolism, hypovolemia.
From the respiratory system
Uncommon: nosebleeds.
From the digestive system
Often - diarrhea; uncommon - abdominal pain, flatulence, polydipsia; frequency is unknown - dry mouth, nausea, vomiting, loss of appetite, pancreatitis, dyspeptic symptoms, intrahepatic cholestasis.
From the kidneys and urinary tract
Often - increased frequency of urination, polyuria, nocturia; infrequently - frequent urge to urinate; frequency unknown - oliguria, urinary retention (in patients with urinary tract obstruction), interstitial nephritis, hematuria, increased concentrations of urea and creatinine in the blood.
From the genital organs
Frequency unknown - impaired potency.
General and administration site disorders
Uncommon: asthenia (exhaustion), thirst, weakness, increased fatigue, hyperactivity, nervousness.
Laboratory indicators
Uncommon: increased platelet count; frequency unknown - hyperglycemia, hyperuricemia, decrease in the number of red blood cells, leukocytes and platelets, a slight increase in the activity of alkaline phosphatase in the blood, increased activity of some liver enzymes (for example, gamma-glutamyl transferase).
From the skin and subcutaneous tissues
Frequency unknown - pruritus, rash, urticaria, erythema multiforme, exfoliative dermatitis, purpura, vasculitis, photosensitivity.
From the musculoskeletal system
Frequency unknown - muscle weakness.
From the side of water-electrolyte and acid-base balance
Frequency not known - hyponatremia, hypochloremia, hypokalemia, hypomagnesemia, hypocalcemia, metabolic alkalosis. Symptoms indicating the development of electrolyte and acid-base disorders may include headache, confusion, convulsions, tetany, muscle weakness, cardiac arrhythmias and dyspeptic disorders; hypovolemia and dehydration (more often in elderly patients), which can lead to hemoconcentration with a tendency to develop thrombosis.
If any of the side effects indicated in the instructions get worse , or you notice any other side effects not listed in the instructions, tell your doctor.
Instructions for use of Torasemide
Instructions for use of Torasemide Sandoz recommends oral (internal) administration of whole tablets in the morning with water.
The duration of administration depends on the nature of the painful condition (in case of heart failure , therapy is continued until the swelling disappears).
For arterial hypertension , the initial dose of torasemide for adults is 2.5 mg per 24 hours. In case of insufficient reduction in blood pressure after 2 months of treatment, the daily dose may be increased to 5 mg. Maximum effectiveness is usually observed after 3 months of therapy. Doses of the drug above 5 mg do not provide additional antihypertensive effect .
To prevent or reduce edema , it is recommended to begin treatment with torasemide with a daily dose of 5 mg, which, if necessary, is doubled (10 mg). For severe edema, a gradual increase in the daily dose to 20 mg is possible.
Liver cirrhosis requires the use of a total initial daily dose of 5 to 10 mg, with parallel administration of aldosterone or diuretics that promote potassium retention in the body. In the absence of a sufficient diuretic effect, the dose is doubled (from 10 to 20 mg).
For chronic renal failure , the dosage of Torasemide Sandoz is determined individually depending on the degree of the disease state. Typically, the initial daily dose of the drug is 20 mg and, if necessary, is gradually increased up to a dose of 200 mg per 24 hours, which is the maximum. This dose is prescribed only in cases of severe renal dysfunction with CC less than 20 ml/min, as well as during hemodialysis , in the presence of diuresis of at least 200 ml per day.
TORESEMIDE (tablets)
ie.
Doctors shrug their shoulders - many years of serious illness and age. On her last visit, the doctor prescribed my mother a new drug, Torasemide-3C, which, according to her, is very effective and has a number of advantages over Furosemide. Compared to Furosemide, the diuretic drug Torasemide is less active in reducing the level of potassium in the blood, has a longer and more powerful effect, and its duration of action is up to 18 hours. And yet, unlike Furosemide, the drug Torasemide can be taken for a long time without a threat to health. The doctor prescribed taking the drug Torasemide at a dose of 5 mg tablet once a day, regardless of meals, with a sufficient amount of water, but at the same time. And she also warned that due to the diuretic effect of the drug, blood pressure decreases, so it needs to be constantly monitored. According to the doctor, with constant use of Torasemide, the dose will gradually decrease over time and in order to adjust the blood pressure, you need to correctly correlate the antihypertensive drug you are taking with Torasemide. It’s too early to talk about this now, it takes time, but we are constantly monitoring the pressure. The diuretic drug “Torasemide-C3”, which I bought in a dose of 10 mg (again, I only took one plate so far) costs 240 rubles - 60 tablets, “Leningrad region . The pharmacist gave me a plate of the drug (10 tablets) with packaging and instructions (there was one blister left in the package). The box is informative, the necessary information is given on all sides. By the way, on one of the sides it is indicated that the drug is dispensed by prescription - nothing like that! I decided to look through the entire instructions, but there is so much information there that my head is spinning. This is correct, but the doctor must delve into all this and be interested in it so as not to harm the patient. It is indicated that it should be taken only as prescribed by a doctor.
Here are the indications for the use of “Torasemide”, contraindications and who to take with caution: “Torasemide” tablets are white, the score is divided in half, so it is convenient to take a larger dosage - it turns out cheaper. We noticed that after just 4-5 days of taking Torasemide, the swelling in the legs decreased and breathing became easier, and this is already an achievement. And yet, after taking Torasemide, you don’t have to run to the toilet every minute, like with Furosemide. All this happens less frequently, smoothly over 15-18 hours. This is convenient, especially for bed patients. I hope that in the future the situation will become even better. The record is running out, we'll take the next one. I am very glad that the drug improved my mother’s general condition, and most importantly, shortness of breath decreased and breathing became easier.
I recommend the drug “Torasemide”, the drug is good and the price is affordable. The only thing is that self-medication is unacceptable, especially in severe cases, so take the drug only as directed and under the supervision of a doctor. Do not be ill!
Overdose
Typical symptoms of torasemide are unknown. Possible forced diuresis with excessive loss of electrolytes and fluid, drowsiness , arterial hypotension , confusion, digestive tract disorders, cardiovascular failure .
specific antidote . Depending on the manifestations of overdose, a reduction in the dose taken or complete withdrawal of the drug is indicated. It is recommended to take measures to compensate for losses in water and electrolyte balance. Hemodialysis is ineffective.
In case of hypovolemia, the volume of lost fluid is replaced. For hypokalemia, potassium supplements are prescribed. If cardiovascular failure , the patient is placed in a sitting position and given symptomatic treatment.
At the first occurrence of skin reactions (for example, redness of the skin or urticaria ), headache , agitation, increased sweating , cyanosis , nausea, venous catheterization is recommended. The patient is placed in a horizontal position, a free flow of air is provided, and oxygen is prescribed. If necessary, Epinephrine , glucocorticoids and solutions to replace fluid loss are administered.
AUDITOR (Torasemide) tab 5mg N30
To classify the frequency of adverse reactions, the following categories were used: very often (>1/10), often (>1/100, 1/1000, 1/10000,
Metabolic and nutritional disorders: often - progression of metabolic alkalosis; muscle spasm (especially at the beginning of therapy); increased levels of uric acid, glucose and blood lipids (triglycerides, cholesterol); hypokalemia in combination with a diet low in potassium, with vomiting, diarrhea, with excessive use of laxatives, as well as in patients with chronic liver dysfunction.
Depending on the dose and duration of treatment, fluid and electrolyte imbalances may occur, especially with hypovolemia, hypokalemia and/or hyponatremia.
Disorders of the hematopoietic and lymphatic systems: very rarely - a decrease in the number of platelets, red blood cells and/or leukocytes.
Disorders of the cardiovascular system: very rarely - due to increased blood concentrations, thromboembolic complications, confusion, hypotension, as well as cardiac and central circulatory disorders (including cardiac and cerebral ischemia) may occur. This can lead to, for example, arrhythmia, angina, acute myocardial infarction or fainting.
Gastrointestinal disorders: often - gastrointestinal disorders (for example, lack of appetite, stomach pain, nausea, vomiting, diarrhea, constipation), especially at the beginning of treatment. Very rarely - pancreatitis.
Renal and urinary tract disorders: uncommon - increased levels of creatinine and urea in the blood. In patients with urinary problems (eg, due to prostatic hyperplasia), increased urine production may lead to urinary retention and increased bladder capacity.
Disorders of the liver and biliary tract: often - an increase in the level of certain liver enzymes (gamma-GT).
Skin and subcutaneous tissue disorders: very rare: allergic reactions (eg, itching, exanthema, photosensitivity), serious skin reactions.
General disorders: often - headache, dizziness, fatigue, asthenia (especially at the beginning of therapy). Uncommon: xerostomia, paresthesia. Very rarely: visual impairment, ringing in the ears, hearing loss.
Attention
Long-term use of the drug requires regular monitoring of the level of electrolytes in the blood serum, especially potassium.
It is also necessary to regularly monitor the levels of glucose, uric acid, creatinine and blood lipids.
Due to a possible increase in blood sugar, it is recommended to carefully monitor carbohydrate balance in patients with latent and overt diabetes mellitus. Blood tests (red blood cells, white blood cells, platelets) should be performed regularly. It is important to know the signs of decreased electrolyte levels and blood thickening, especially at the beginning of treatment and in elderly patients.
Interaction
Concomitant use of torasemide and cardiac glycosides may increase the sensitivity of the myocardium to these drugs due to the observed deficiency of magnesium or potassium.
Torsemide enhances the effects of other antihypertensive drugs , including ACE inhibitors , which can cause severe hypotension . To prevent the development of hypotension, the dose of one or another drug should be adjusted.
When combined with laxatives , glucocorticoids and mineralocorticoids, the risk of developing potassium deficiency increases.
Torsemide reduces the effectiveness of antidiabetic drugs and weakens the vasoconstrictor effect of Norepinephrine and Epinephrine .
High doses of torasemide can potentiate the ototoxic and nephrotoxic effects aminoglycoside antibiotics Kanamycin , tobramycin , Gentamicin ), the nephrotoxic effect of cephalosporins and the toxic effect of platinum drugs .
Torsemide increases the effectiveness of curare-like muscle relaxants and Theophylline .
NSAIDs and Probenecid weaken the hypotensive and diuretic effects of torsemide .
The combined use of lithium and torasemide may lead to an increase in lithium levels in the blood, which will increase its neurotoxicity and cardiotoxicity.
Torsemide enhances the toxic effects of salicylates (in high doses) on the central nervous system.
Concomitant use of cholestyramine may reduce the absorption of torasemide , which leads to a weakening of its effect.
Torasemide-SZ tablets 5 mg No. 60
Compound
1 tablet contains: dosage 5 mg: active substance: torasemide – 5 mg excipients: lactose monohydrate (lactopress) (milk sugar) – 89.3 mg; pregelatinized starch (starch 1500) – 24.5 mg; colloidal silicon dioxide (Aerosil) – 0.6 mg; magnesium stearate – 0.6 mg. dosage 10 mg: active substance: torasemide – 10 mg excipients: lactose monohydrate (lactopress) (milk sugar) – 178.6 mg; pregelatinized starch (starch 1500) – 49.0 mg; colloidal silicon dioxide (Aerosil) – 1.2 mg; magnesium stearate – 1.2 mg.
Pharmacokinetics
After oral administration, torasemide is quickly and almost completely absorbed from the gastrointestinal tract. Food intake does not have a significant effect on the absorption of the drug. The maximum concentration of torasemide in the blood plasma is observed 1-2 hours after oral administration. Bioavailability is 80 – 90% with minor individual variations. The diuretic effect lasts up to 18 hours, which facilitates the tolerability of therapy due to the absence of very frequent urination in the first hours after taking the drug orally, which limits the activity of patients. Communication with blood plasma proteins is more than 99%. The apparent volume of distribution is 16 l. Metabolized in the liver using isoenzymes of the cytochrome P450 system. As a result of sequential reactions of oxidation, hydroxylation or ring hydroxylation, three metabolites are formed (M1, M3 and M5), which bind to plasma proteins by 86%, 95% and 97%, respectively. The half-life (T1/2) of torasemide and its metabolites is 3 to 4 hours and does not change in chronic renal failure. The total clearance of torasemide is 40 ml/min, renal clearance is 10 ml/min. On average, about 83% of the dose taken is excreted by the kidneys: unchanged (24%) and in the form of predominantly inactive metabolites (M1 - 12%, M3 - 3%, M5 - 41%). In renal failure, T1/2 does not change, T1/2 of metabolites M3 and M5 increases. Torsemide and its metabolites are slightly eliminated by hemodialysis and hemofiltration. In case of liver failure, the concentration of torasemide in the blood plasma increases due to a decrease in the metabolism of the drug in the liver. In patients with cardiac or liver failure, T1/2 of torasemide and the M5 metabolite is slightly increased, drug accumulation is unlikely.
Indications for use
Edema syndrome of various origins, including with chronic heart failure, diseases of the liver, lungs and kidneys; - arterial hypertension.
Contraindications
Hypersensitivity to torsemide or to any of the components of the drug; in patients with an allergy to sulfonamides (sulfonamide antimicrobials or sulfonylureas); renal failure with anuria; hepatic coma and precoma; refractory hypokalemia/refractory hyponatremia; hypovolemia (with or without arterial hypotension) or dehydration; pronounced disturbances in the outflow of urine of any etiology (including unilateral damage to the urinary tract); glycoside intoxication; acute glomerulonephritis; decompensated aortic and mitral stenosis, hypertrophic obstructive cardiomyopathy; increased central venous pressure (over 10 mm Hg); hyperuricemia; simultaneous use of aminoglycosides and cephalosporins; age under 18 years; pregnancy, breastfeeding period; lactose intolerance, lactase deficiency or glucose-galactose malabsorption. With caution Arterial hypotension, stenosing atherosclerosis of the cerebral arteries, hypoproteinemia, predisposition to hyperuricemia, urinary outflow disorders (benign prostatic hyperplasia, narrowing of the urethra or hydronephrosis), history of ventricular arrhythmia, acute myocardial infarction (increased risk of cardiogenic shock), diarrhea, pancreatitis, diabetes mellitus (decreased glucose tolerance), hepatorenal syndrome, gout, anemia; simultaneous use of cardiac glycosides, corticosteroids and adrenocorticotropic hormone (ACTH).
Directions for use and doses
Orally, once a day, without chewing, with a sufficient amount of water. The tablets can be taken at any convenient time, regardless of meals. Edema syndrome in chronic heart failure The usual starting dose is 10-20 mg once a day. If necessary, the dose can be doubled until the desired effect is obtained. Edema syndrome in kidney disease The usual starting dose is 20 mg once daily. If necessary, the dose can be doubled until the desired effect is obtained. Edema syndrome in liver disease The usual starting dose is 5-10 mg once daily. If necessary, the dose can be doubled until the desired effect is obtained. The maximum single dose is 40 mg; it is not recommended to exceed it (there is no experience with use). The drug is used for a long period or until swelling disappears. Arterial hypertension The initial dose is 2.5 mg (1/2 tablet of 5 mg) once a day. If there is no therapeutic effect within 4 weeks, the dose is increased to 5 mg once a day. If there is no adequate reduction in blood pressure when taken at a dose of 5 mg once a day for 4-6 weeks, the dose is increased to 10 mg once a day. If the use of the drug at a dose of 10 mg per day does not give the required effect, an antihypertensive drug of another group is added to the treatment regimen. Elderly patients do not require dose adjustment.
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 oC. Keep out of the reach of children.
Best before date
3 years. Do not use after the expiration date stated on the packaging.
special instructions
Use strictly as prescribed by your doctor. Patients with hypersensitivity to sulfonamides and sulfonylurea derivatives may have cross-sensitivity to Torasemide-SZ. For patients receiving high doses of Torasemide-SZ for a long period, in order to avoid the development of hyponatremia, metabolic alkalosis and hypokalemia, a diet with sufficient salt content and the use of potassium supplements are recommended. An increased risk of developing fluid and electrolyte imbalances is observed in patients with renal failure. During the course of treatment, it is necessary to periodically monitor the concentration of blood plasma electrolytes (including sodium, calcium, potassium, magnesium), acid-base status, residual nitrogen, creatinine, uric acid and, if necessary, carry out appropriate corrective therapy (with a higher frequency in patients with frequent vomiting and against the background of parenterally administered fluids). If azotemia and oliguria appear or worsen in patients with severe progressive kidney disease, it is recommended to suspend treatment. The selection of a dosage regimen for patients with ascites against the background of liver cirrhosis should be carried out in a hospital setting (violations of water and electrolyte balance can lead to the development of hepatic coma). This category of patients requires regular monitoring of blood plasma electrolytes. In patients with diabetes mellitus or with reduced glucose tolerance, periodic monitoring of glucose concentrations in the blood and urine is required. In unconscious patients with prostatic hyperplasia and narrowing of the ureters, diuresis control is necessary due to the possibility of acute urinary retention.
Description
Tablets are white or almost white, round, flat-cylindrical with a chamfer and a score on one side.
Conditions for dispensing from pharmacies
Dispensed by prescription.
Dosage form
Pills
Manufacturer and organization accepting consumer complaints
188663, Leningrad region, Vsevolozhsk district, urban settlement. Kuzmolovsky, workshop building No. 188 tel/fax
Pharmacodynamics
Torsemide is a loop diuretic. The maximum diuretic effect develops 2-3 hours after taking the drug orally. The main mechanism of action of the drug is due to the reversible binding of torasemide to the sodium/chlorine/potassium ion contransporter located in the apical membrane of the thick segment of the ascending loop of Henle, as a result of which the reabsorption of sodium ions is reduced or completely inhibited and the osmotic pressure of intracellular fluid and water reabsorption are reduced. Blocks myocardial aldosterone receptors; reduces fibrosis and improves diastolic myocardial function. Torasemide causes hypokalemia to a lesser extent than furosemide, but it is more active and its action lasts longer. The use of torasemide is the most reasonable choice for long-term therapy.
Side effects
The frequency of side effects is classified according to the recommendations of the World Health Organization: very often: ? 1/10 (> 10%); often: from ? 1/100 to 1% and 0.1% and 0.01% and <0.1%)); very rare: <1/10000 (<0.01%); frequency unknown: frequency cannot be estimated from available data. From the nervous system: often – headache, dizziness, drowsiness; infrequently – muscle cramps of the lower extremities; frequency unknown - confusion, fainting, paresthesia in the extremities (feeling of numbness, “crawling” and tingling). From the senses: frequency unknown - visual impairment, hearing impairment, tinnitus and hearing loss (usually reversible), usually in patients with renal failure or hypoproteinemia (nephrotic syndrome). From the cardiovascular system: infrequently - extrasystole, arrhythmia, tachycardia; frequency unknown - excessive decrease in blood pressure, orthostatic hypotension, collapse, deep vein thrombosis, thromboembolism, decrease in circulating blood volume. From the respiratory system: infrequently - nosebleeds. From the digestive system: often – diarrhea; uncommon – abdominal pain, flatulence, polydipsia; frequency unknown - dry mouth, nausea, vomiting, loss of appetite, pancreatitis, dyspeptic disorders, intrahepatic cholestasis. From the skin and subcutaneous tissues: frequency unknown - skin itching, rash, urticaria, erythema multiforme, exfoliative dermatitis, purpura, vasculitis, photosensitivity. From the musculoskeletal system: frequency unknown - muscle weakness. From the urinary system: often - increased frequency of urination, polyuria, nocturia; infrequently – frequent urge to urinate; frequency unknown - oliguria, urinary retention (in patients with urinary tract obstruction), interstitial nephritis, hematuria. From the reproductive system: frequency unknown – decreased potency. Metabolic: frequency unknown - hypokalemia, hyponatremia, hypomagnesemia, hypocalcemia, hypochloremia, metabolic alkalosis, hypovolemia, dehydration (more often in elderly patients). From laboratory parameters: infrequently – hypercholesterolemia, hypertriglyceridemia; frequency unknown - hyperuricemia, a slight increase in the activity of alkaline phosphatase in the blood plasma, an increase in the concentration of creatinine and urea in the blood plasma, an increase in the activity of some “liver” enzymes in the blood plasma (for example, gamma-glutamyltransferase), thrombocytopenia, leukopenia, agranulocytosis, hyperglycemia, decrease glucose tolerance (possible manifestation of latent diabetes mellitus). Other: frequency unknown - aplastic or hemolytic anemia.
Use during pregnancy and breastfeeding
Torsemide does not have a teratogenic effect or fetotoxicity; it penetrates the placental barrier, causing disturbances in water-electrolyte metabolism and thrombocytopenia in the fetus. There have been no controlled studies on the use of torasemide in pregnant women; the drug is not recommended for use during pregnancy. It is unknown whether torasemide passes into breast milk. If it is necessary to use the drug Torasemide-SZ during lactation, you must stop breastfeeding.
Interaction
Increases the concentration and risk of developing nephro- and ototoxic effects of cephalosporins, aminoglycosides, chloramphenicol, ethacrynic acid, cisplatin, amphotericin B (due to competitive renal excretion). Increases the effectiveness of diazoxide and theophylline, reduces the effectiveness of hypoglycemic agents, allopurinol. Pressor amines and torasemide mutually reduce effectiveness. Drugs that block tubular secretion increase the concentration of torasemide in the blood serum. With the simultaneous use of glucocorticosteroids, amphotericin B, the risk of developing hypokalemia increases, with cardiac glycosides - the risk of developing glycoside intoxication increases due to hypokalemia (for high- and low-polarity) and prolongation of the half-life (for low-polarity). Reduces the renal clearance of lithium drugs and increases the likelihood of intoxication. Non-steroidal anti-inflammatory drugs, sucralfate, reduce the diuretic effect due to inhibition of prostaglandin synthesis, impaired renin activity in the blood plasma and the excretion of aldosterone. Strengthens the antihypertensive effect of antihypertensive drugs, neuromuscular blockade of depolarizing muscle relaxants (suxamethonium) and weakens the effect of non-depolarizing muscle relaxants (tubocurarine). Concomitant use of large doses of salicylates during torsemide therapy increases the risk of their toxicity (due to competitive renal excretion). Sequential or simultaneous use of torasemide with angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor antagonists can lead to a severe decrease in blood pressure. This can be avoided by reducing the dose of torasemide or temporarily stopping it. Concomitant use of probenecid or methotrexate may reduce the effectiveness of torsemide (same secretion route). On the other hand, torasemide may lead to decreased renal elimination of these drugs. With the simultaneous use of cyclosporine and torasemide, the risk of developing gouty arthritis increases due to the fact that cyclosporine can cause impaired urate excretion by the kidneys, and torasemide can cause hyperuricemia. It was reported that in patients at high risk of developing nephropathy taking torasemide orally, renal dysfunction was observed more frequently when radiocontrast agents were administered than in patients at high risk of developing nephropathy who were given intravenous hydration before administration of radiocontrast agents. The bioavailability and, as a consequence, the effectiveness of torasemide may be reduced when combined with cholestyramine.
Overdose
Symptoms: increased diuresis, accompanied by a decrease in circulating blood volume and disruption of the water-electrolyte balance of the blood, followed by a pronounced decrease in blood pressure, drowsiness and confusion, collapse. Gastrointestinal disturbances may occur. Treatment: there is no specific antidote. Provocation of vomiting, gastric lavage, activated charcoal. Treatment is symptomatic, dose reduction or discontinuation of the drug and at the same time replenishment of blood volume and indicators of water-electrolyte balance and acid-base status under the control of serum concentrations of electrolytes, hematocrit, symptomatic treatment. Hemodialysis is ineffective.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, patients should refrain from driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions (risk of dizziness and drowsiness).
special instructions
Before prescribing therapy with Torasemide Sandoz, the concentrations of sodium and potassium, as well as the volume of circulating blood, should be normalized.
During long-term treatment, it is recommended to conduct regular studies of electrolyte balance (especially in patients simultaneously using glucocorticoids , digitalis glycosides , laxatives, mineralocorticosteroids ), glucose, creatinine, uric acid and blood lipids.
Patients with diabetes should monitor carbohydrate metabolism.
Patients with a tendency to develop gout and hyperuricemia require special attention from a doctor.
Torsemide is not recommended for patients with pathological changes in acid-base balance; altered blood picture ( anemia , thrombocytopenia , etc.) in patients without renal failure; in combination with lithium, cephalosporins and aminoglycosides ; children; for kidney pathologies due to the intake of nephrotoxic substances; in old age.
Torsemide Sandoz requires special caution when prescribing to patients with liver pathologies accompanied by ascites and cirrhosis , since sharp fluctuations in water and electrolyte balance can cause hepatic coma . In this regard, treatment with diuretics should be carried out in a hospital. To prevent manifestations of metabolic acidosis and hypokalemia, parallel administration of aldosterone antagonists or potassium-sparing drugs is necessary.
Sometimes, after taking torasemide, ototoxicity phenomena (hearing loss, tinnitus) were observed, which were reversible and without a proven cause-and-effect relationship.
Diuretic therapy requires constant monitoring of electrolyte balance, extrarenal azotemia, hypovolemia and other disorders, symptoms of which may include: dry mouth , thirst, lethargy, weakness, drowsiness , tachycardia , muscle cramps or pain, agitation, myasthenia gravis, oliguria, hypotension, nausea , turning into vomiting. High diuresis can lead to dehydration and a decrease in the amount of circulating blood, embolism and thrombus formation in blood vessels, especially in elderly patients.
In case of water and electrolyte disturbances, it is necessary to interrupt therapy, with the possibility of continuing it in lower doses after the elimination of negative symptoms.
Laboratory monitoring of plasma levels of potassium and other electrolytes should be carried out regularly.
Tablets of the drug from 2.5 mg to 20 mg include lactose , which should be taken into account when prescribing the drug to patients with sugar intolerance .
Tablets ranging from 50 mg to 200 mg contain hydrogenated castor oil , which may cause gastrointestinal upset and diarrhea .
THORASEMIDE
Side effects
The frequency of the side effects listed below was determined according to the following (World Health Organization classification): very common (more than 10%);
often (more than 1% and less than 10%); uncommon (more than 0.1% and less than 1%); rare (more than 0.01% and less than 0.1%); very rarely (less than 0.01%), including isolated reports; frequency unknown (cannot be estimated from available data). From the blood and lymphatic system
Frequency unknown
- thrombocytopenia, leukopenia, agranulocytosis, aplastic or hemolytic anemia.
Metabolism and nutrition
Infrequently
- hypercholesterolemia, hypertriglyceridemia.
Frequency unknown
- decreased glucose tolerance (possible manifestation of latent diabetes mellitus).
From the nervous system
Often -
dizziness, headache, drowsiness;
infrequently -
muscle cramps of the lower extremities;
frequency is unknown
- confusion, fainting, paresthesia in the extremities (feeling of numbness, “crawling” and tingling).
From the side of the organ of vision
Frequency unknown -
visual impairment.
Hearing and labyrinth disorders
Frequency not known? 1a —
Hearing impairment, tinnitus and hearing loss (usually reversible) usually occur in patients with renal failure or hypoproteinemia (nephrotic syndrome).
From the cardiovascular system
Infrequently -
extrasystole, tachycardia, increased heartbeat, facial flushing;
frequency not known -
excessive arterial hypotension, orthostatic hypotension, collapse, deep vein thrombosis, thromboembolism, hypovolemia.
From the respiratory system
Infrequently -
nosebleeds.
From the digestive system
Often
- diarrhea;
uncommon
- abdominal pain, flatulence, polydipsia;
frequency is unknown -
dry mouth, nausea, vomiting, loss of appetite, pancreatitis, dyspeptic symptoms, intrahepatic cholestasis.
From the kidneys and urinary tract
Often -
increased frequency of urination, polyuria, nocturia;
infrequently -
frequent urge to urinate;
frequency unknown
- oliguria, urinary retention (in patients with urinary tract obstruction), interstitial nephritis, hematuria, increased concentrations of urea and creatinine in the blood.
From the genital organs
Frequency unknown
- violation of potency.
General and administration site disorders
Infrequently
- asthenia (exhaustion), thirst, weakness, increased fatigue, hyperactivity, nervousness.
Laboratory indicators
Infrequently -
increase in platelet count;
frequency unknown -
hyperglycemia, hyperuricemia, decrease in the number of red blood cells, leukocytes and platelets, a slight increase in the activity of alkaline phosphatase in the blood, increased activity of some liver enzymes (for example, gamma-glutamyl transferase).
From the skin and subcutaneous tissues
Frequency unknown
- skin itching, rash, urticaria, erythema multiforme, exfoliative dermatitis, purpura, vasculitis, photosensitivity.
From the musculoskeletal system
Frequency unknown
- muscle weakness.
From the side of water-electrolyte and acid-base balance
Frequency unknown
- hyponatremia, hypochloremia, hypokalemia, hypomagnesemia, hypocalcemia, metabolic alkalosis. Symptoms indicating the development of electrolyte and acid-base disorders may include headache, confusion, convulsions, tetany, muscle weakness, cardiac arrhythmias and dyspeptic disorders; hypovolemia and dehydration (more often in elderly patients), which can lead to hemoconcentration with a tendency to develop thrombosis.
If any of the side effects indicated in the instructions get worse ,
or you notice any other side effects not listed in the instructions, tell your doctor.
Torasemide's analogs
Level 4 ATC code matches:
Lasix
Furosemide
Britomar
Trigrim
Trifas
Diuver
Analogues of Torasemide Sandoz are represented by the following drugs: Furosemide , Lasix and Bufenox .
Torasemide Sandoz price, where to buy
The average price of Torasemide in Russia is:
- 10 mg No. 20 - 900 rubles;
- 20 mg No. 20 - 1300 rubles;
- 100 mg No. 20 - 4000 rubles;
- 200 mg No. 20 - 6000 rubles.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
LuxPharma* special offer
- Torasemide 5mg/ml 4ml ampoules No. 5
RUB 1,390 order
ZdravCity
- Torasemid-SZ tablets 10 mg 60 pcs. North Star JSC
RUB 367 order
- Torasemide Canon tablets 10 mg 20 pcs. JSC Kanonpharma Production
206 rub. order
- Torasemide Medisorb tablets 10 mg 20pcs JSC Medisorb
189 rub. order
- Torasemid-SZ tablets 5 mg 30 pcs. North Star JSC
RUB 161 order
- Torasemide tablets 10mg 20pcsOzon LLC
RUB 214 order
Pharmacy Dialogue
- Torasemid-SZ (tab. 10 mg No. 30) North Star ZAO
210 rub. order
- Torasemid Canon (5 mg tablet No. 20)Canonpharma Production
RUB 152 order
- Torasemide (5 mg tablet No. 60) Berezovsky plant
RUB 223 order
- Torasemid (tab. 10 mg No. 60) Berezovsky plant
RUB 279 order
- Torasemide Canon tablets 10 mg No. 20Canonpharma Production
182 RUR order
show more
Pharmacy24
- Torasemid-Teva 5 mg No. 20 tablets Pliva Hrvatska d.o.o., Croatia
75 UAH.order - Torasemid-Teva 5 mg No. 30 tablets Pliva Hrvatska d.o.o., Croatia
108 UAH order
- Torasemide Sandoz 20 mg No. 100 tablets Salutas Pharma GmbH, Nimechchina / Lek S.A. Poland
652 UAH.order
- Torasemide Sandoz 20 mg No. 20 tablets Salutas Pharma GmbH, Nimechchina / Lek S.A. Poland
143 UAH order
- Torasemide Sandoz 50 mg No. 20 tablets Salutas Pharma GmbH, Nimechchina / Lek S.A. Poland
330 UAH. order