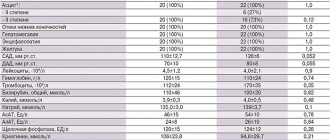
To improve or maintain metabolism, as well as energy supply to all organs of the human body, experts prescribe Mildronate.
The good effect, as well as the few side effects of Mildronate, determine its popularity among almost all categories of the population.
Like any other drug, Mildronate should be prescribed by a doctor even in the absence of visible symptoms of the disease.
The active ingredient of the drug is meldonium, which leads to:
- to improve the body's performance;
- increasing humoral and tissue immunity;
- increasing human resistance to negative psychological and physiological factors;
- normalization of cardiac activity.
In addition, the product protects cells from destruction, removes toxic products and waste from cellular metabolism, and also helps to increase their stability. As a result, the increased metabolic rate leads to faster recovery of the body.
The drug's ability to optimize blood flow allows it to redirect blood flow to oxygen-starved areas of the heart, brain and eye. As a result, blood supply is normalized, the organ receives a sufficient amount of oxygen and necessary nutrients. This property of Mildronate is of particular relevance in ischemia.
The use of Mildronate leads to vasodilation and normalization of cellular immunity, as a result of which immunity in general improves.
The tonic effect on the somatic and autonomic nervous system leads to the elimination of physical and psychological disorders due to prolonged use of alcoholic beverages. Allows you to eliminate alcohol withdrawal, manifested in the form of tremors, memory loss, and obsessive states.
Among the most common indications for the use of Mildronate are:
- ischemia of the heart and brain;
- angina pectoris;
- heart attack;
- chronic heart failure;
- pain syndrome in the heart due to pathologies of the organ;
- dishormonal cardiomyopathy;
- impaired functioning of the cardiovascular system and neurocirculatory dystonia during puberty;
- pathological changes in the eyes;
- chronic obstructive pulmonary pathologies;
- psychological and physical consequences of long-term alcohol use;
- low performance;
- overstrain and fatigue.
Only a doctor can determine the need to use the drug
Contraindications to the use of the drug
The drug's few contraindications make it suitable for use in almost all categories of patients.
The exceptions are:
- Pregnant women. The lack of clinical studies on the effect of the drug on the fetus and the woman’s health makes its use undesirable while expecting a child.
- Breastfeeding period. At the moment, there is no information about the penetration of the substance into the milk of a nursing woman, therefore, it is impossible to assess the possible harm when taking it on the health of the child. During lactation, use of the drug is not recommended.
- Individual intolerance. Hypersensitivity to meldonium, as well as to other ingredients of the product, makes it impossible to use.
- With an excessive increase in intracranial pressure due to impaired venous outflow or tumor processes in the brain.
- Age category up to 12 years. In pediatrics, there is not enough information about the effect of Mildronate on the child’s condition. There is no categorical ban on its use by children.
- Nitroglycerin, Nifedipine, adrenergic blockers when used together with Mildronate can provoke an increase in heart rate or a decrease in blood pressure.
- Pathological changes in the liver and kidneys, which are chronic in nature, necessitate the use of the drug with caution, constantly monitoring the condition of the affected organ.
- Edema of unknown origin.
Even if there are no contraindications to the drug indicated in the instructions, you should not resort to the drug without first consulting a doctor.
In addition, the stimulating effect of the drug forces it to be used well before bedtime in order to avoid insomnia.
It is also prohibited to use the drug for intramuscular administration. The instructions clearly indicate that the product is intended for intravenous administration in the form of an injection solution and oral administration in the form of capsules.
If Mildronate is injected into a muscle, such an action is highly likely to cause irritation and provoke the development of a local inflammatory process with severe pain. Allergic reactions also often occur at the site of injection into the muscle tissue.
Mildronate is contraindicated in patients with severe renal impairment (renal failure). Since the drug is excreted from the body through the kidneys, it is better for people with their diseases to refuse such treatment. In cases of mild to moderate impairment of renal excretory function, the drug can be used, but in a lower dose than recommended.
Treatment with meldonium in patients with severe liver dysfunction (hepatocellular insufficiency) is also prohibited, since the drug is metabolized in the liver. And in case of disruption of the activity of liver cells, it is unknown how this process occurs and what the consequences may be (this aspect of the use of meldonium has not been studied in clinical studies).
Restrictions on the use of the drug also apply to elderly patients. Since many elderly people have several chronic diseases, including pathologies of the liver and kidneys, caution should be exercised when prescribing any medications, including Mildronate. If it is not known about the presence of the above contraindications to the use of the drug, then the medication can be prescribed for older people, but in less than the recommended dose.
Before using the drug, be sure to study the instructions and contraindications therein.
Instructions for use MILDRONATUM
A drug that improves metabolism. It is a structural analogue of the carnitine precursor gamma-butyrobetaine (GBB), in which one hydrogen atom is replaced by a nitrogen atom.
It is assumed that there are 2 mechanisms of action of Mildronate on the body
1. Effect on carnitine synthesis
As a result of inhibition of the activity of butyrobetaine hydroxylase, Mildronate reduces the biosynthesis of carnitine and inhibits the transport of long-chain fatty acids through the cell membrane, preventing the accumulation in cells of activated derivatives of unoxidized fatty acids - acylcarnitine and acyl coenzyme A, which have pronounced detergent properties. Under ischemic conditions, Mildronate restores the balance between oxygen delivery and consumption in cells, eliminates disturbances in ATP transport, while simultaneously activating an alternative energy source - glycolysis, which occurs without additional oxygen consumption.
With increased load, as a result of intensive energy consumption, a temporary decrease in the content of fatty acids, mainly carnitine, occurs in the cells of a healthy body. It is known that carnitine biosynthesis is regulated by its level in the blood plasma and stress, but does not depend on the concentration of carnitine precursors in the cell. Since Mildronate inhibits the conversion of GBB to carnitine, this leads to a decrease in the level of carnitine in the blood, which in turn activates the process of synthesis of the carnitine precursor - GBB.
When the concentration of Mildronate decreases, the process of carnitine biosynthesis is restored and the concentration of fatty acids in the cell is normalized. Thus, the cells undergo regular training, which contributes to their survival under conditions of increased load, in which the content of fatty acids in them regularly decreases, and when the load decreases, the fatty acid content is quickly restored. Under real overload conditions, cells “trained” with Mildronate survive in conditions where “untrained” cells die.
2. Function of the mediator of the hypothetical GBB-ergic system
It has been hypothesized that in the body there is a previously undescribed system for the transmission of nerve impulses - the GBB-ergic system, which ensures the transmission of nerve impulses to somatic cells. The mediator of this system is the immediate precursor of carnitine, the GBB ester. As a result of the action of esterase, this transmitter gives the cell an electron, thus transferring an electrical impulse, and itself turns into GBB.
GBB synthesis is possible in any somatic cell of the body. Its speed is regulated by the intensity of the stimulus and energy expenditure, which in turn depend on the concentration of carnitine. Therefore, when carnitine concentration decreases, GBB synthesis is stimulated. When the concentration of a mediator (GBB ester) increases in any place in the body, with the participation of the central nervous system, regulatory mechanisms are activated that are “responsible” specifically for it.
Thus, the body has an economical chain of reactions that provides an adequate response to irritation or stress:
- it begins with the receipt of a signal from nerve fibers (in the form of an electron), followed by the synthesis of GBB and its ester, which, in turn, transfers the signal to the membranes of somatic cells. Somatic cells, in response to irritation, synthesize new molecules, ensuring the propagation of the signal. After this, the hydrolyzed form of GBB, with the participation of active transport, enters the liver, kidneys and testes, where it is converted into carnitine.
As stated earlier, Mildronate is a structural analogue of GBB, in which one hydrogen atom is replaced by a nitrogen atom. Since Mildronate can be affected by GBB esterase, it may function as a hypothetical "mediator". However, GBB hydroxylase does not act on mildronate and therefore, when it is introduced into the body, the concentration of carnitine does not increase, but decreases. Due to the fact that Mildronate itself acts as a “mediator” of stress and also increases the content of GBB, it contributes to the development of the body’s response. As a result, overall metabolic activity increases in other systems, for example, the central nervous system.
Experimental results
In experiments on anesthetized cats, the effect of mildronate on indicators of the cardiovascular system was studied. It has been established that over a wide range of doses, the drug increases blood flow, systolic and minute volumes, does not have a significant effect or slightly reduces venous pressure. These data indicate a positive effect of Mildronate on myocardial contractile function.
Mildronate has a protective effect against myocardial hypoxia. In experiments on isolated rabbit atria, it was found that after the cessation of hypoxic effects, the drug accelerates the restoration of myocardial contractility to a normal level.
In another series of experiments on anesthetized white rats, it was revealed that the administration of Mildronate before the start of the experiment eliminates the increase in ischemia (ST segment elevation) after ligation of the left coronary artery, reduces the corresponding focus of the infarction and increases the life expectancy of the animals. Upon repeated administration of the drug for 10 days, a pronounced (more than 2-fold compared to the control) reduction in the infarction focus was detected.
Mildronate is more effective than nifedipine in preventing the development of heart rhythm disturbances, including ventricular fibrillation, in rats after ligation of the coronary arteries.
In conditions of “lightened” experimental myocardial infarction (ligation of the left coronary artery for ≤1 hour) in dogs, the drug eliminates ischemic ECG changes.
Mildronate protects the myocardium from the damaging effects of catecholamines and alcohol.
In experiments on rats, it was found that preliminary administration of Mildronate in 50% of animals completely eliminates ECG changes caused by isoproterenol (isadrin) and epinephrine (adrenaline). Histological examination of the myocardium of rats indicates that when epinephrine is used, mildronate protects myocardial cells, prevents the development of irreversible changes in cardiomyocytes and promotes the restoration of normal myocardial structure.
Experimental studies have established that myocardial damage caused by toxic concentrations of ethyl alcohol is, in principle, no different from those caused by ischemia or stress. It can be assumed that this is due to the similarity of the mechanisms of action of factors affecting myocardial tissue. The data that Mildronate reduces myocardial damage caused by ethyl alcohol serves as a justification for the use of the drug for the correction of functional and organic disorders of the body in chronic alcohol intoxication.
It has been experimentally established that Mildronate reduces the stimulating effect of ethyl alcohol by approximately 5 times. Under “free choice” conditions, mildronate reduces the consumption of ethyl alcohol by experimental animals by 80-100%.
In animal experiments, the antihypoxic and cerebral blood flow-improving effects of the drug were observed. It has been established that Mildronate has a positive effect in ischemia of brain tissue. The drug optimizes the distribution of cerebral blood flow in favor of ischemic foci and increases the resistance of neurons to hypoxia. Thus, in experiments on rats, the drug partially or completely eliminated EEG changes after occlusion of the carotid artery. Mildronate has an activating effect on the central nervous system:
- motor activity and physical performance increase, stimulation of motor reactions is noted, as well as an anti-stress effect, manifested in stimulation of the sympathetic nervous system, an increase in the content of catecholamines in the brain and adrenal glands, and a protective effect against stress-induced changes in internal organs.
The obtained results of experimental studies indicate the effectiveness of Mildronate and the possibility of its use in cerebrovascular pathology.
Clinical trial results
Analysis of clinical data on the course of treatment of stable angina with Mildronate indicates that under the influence of the drug the frequency and intensity of attacks are reduced, and the amount of nitroglycerin used is also reduced. The drug has a pronounced antiarrhythmic effect in the presence of ischemic heart disease and ventricular extrasystoles and is less effective in supraventricular extrasystoles. Particularly noteworthy is the drug’s ability to reduce oxygen consumption at rest, which is considered an indicator of the effectiveness of antianginal treatment of coronary artery disease. Mildronate has a beneficial effect on atherosclerotic processes in the coronary and other peripheral vessels as a result of reducing the level of total cholesterol in the blood plasma and the atherogenic index.
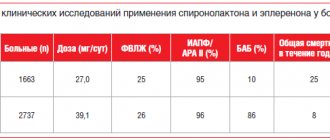
The role of Mildronate in the treatment of chronic heart failure due to coronary artery disease has been analyzed on the basis of a fairly large number of clinical studies, which indicate that the drug increases exercise tolerance and the amount of work performed by patients suffering from heart failure.
In a separate study from the Latvian and Tomsk Institutes of Cardiology, the effectiveness of Mildronate in moderate heart failure (functional class II according to the NYHA classification) was studied. After treatment with Mildronate, 59-78% of patients with an initial diagnosis of functional class II heart failure were assigned to the functional class I group.
It has been established that Mildronate improves myocardial inotropic function and increases exercise tolerance, improves the quality of life of patients without causing severe side effects. It is noted, however, that Mildronate can cause moderate arterial hypotension, allergic skin reactions, headaches, and a feeling of discomfort in the pit of the stomach. In severe heart failure, Mildronate should be prescribed in combination with traditional drugs used to treat this disease.
A good clinical effect of Mildronate was observed in patients with cardialgia that developed as a result of menopause and alimentary obesity.
In clinical settings, it has been established that treatment with Mildronate reduces the frequency of relapses of alcoholism. Mildronate has been shown to relieve alcohol withdrawal syndrome.
Mildronate is an effective means of complex treatment of acute and chronic cerebrovascular accidents (ischemic stroke, chronic cerebral circulatory failure). It has been established that Mildronate normalizes the tone and resistance of capillaries and arterioles of the brain, and restores their reactivity.
The effect of Mildronate on the rehabilitation process of patients with neurological disorders (after cerebrovascular diseases, brain surgery, tick-borne encephalitis) was studied.
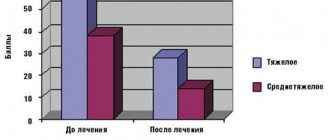
The results of testing the therapeutic effectiveness indicate a positive, dose-dependent effect of the drug on the restoration of physical performance and functional independence of patients during the recovery period. When analyzing changes in individual and integral intellectual functions, a positive effect of Mildronate on the process of restoring intellectual functions during the rehabilitation period was revealed. It was found that Mildronate improves the quality of life of those recovering (mainly as a result of the restoration of physical functions), and also helps to eliminate mental disorders in patients.
During the rehabilitation period, Mildronate has a positive effect on the regression of nervous system dysfunction in patients with neurological deficits. The general neurological condition of patients improves (reduction of brain damage and reflex pathology, regression of paresis, improvement of motor coordination and autonomic functions).
In the clinic and practice of sports medicine, experimental data have been confirmed on the ability of Mildronate to increase performance and accelerate the processes of recovery after intense physical activity. These features of the drug’s action are characterized by improved tolerance to training loads, elimination of the feeling of fatigue, and a surge of vigor. In addition, the drug reduces emotional and mental stress (disappearance of apathy and irritability after exertion in training and competitions).
Toxicological characteristics
Mildronate is low toxic. When the active substance was administered orally to mice and rats, the LD50 value exceeded 18,000 mg/kg. Repeated administration of mildronate to rats and dogs for 6 months did not cause adverse changes in body weight, blood composition, biochemical parameters of blood and urine of animals. In high doses, Mildronate caused hemorrhages in the liver and kidneys in dogs, without affecting the functions of these organs.
When studying the specific toxicity of Mildronate, no teratogenic or embryotoxic effects were observed. The drug does not have mutagenic or carcinogenic properties. In experiments on animals, no allergenic effect was detected.
Known Side Effects
Side effects of Mildronate are not observed often and appear:
- tachycardia;
- surges in blood pressure;
- feeling of nausea and heaviness in the stomach, belching, heartburn, vomiting, constipation, diarrhea;
- allergies: redness of the epidermis, rashes, itching, swelling;
- reaction of the central nervous system: headaches, severe overexcitation.
When taking the drug orally, an overdose is not observed, but it is not excluded with injections and manifests itself in the form of:
- low blood pressure;
- headache;
- dizziness;
- increased heart rate;
- feelings of general weakness.
Side effects and manifestations of overdose are eliminated by stopping the drug and symptomatic treatment.
Mildronate has good reviews and, when used correctly, has a pronounced positive effect.
Side effects of taking Mildronate can be divided depending on the body system that is affected.
Mildronate is a low-toxic drug. Undesirable effects caused by it do not pose a risk to the human condition
The immune system
Often – allergic skin manifestations.
Rarely - urticaria, angioedema, anaphylactic shock.
Human psyche
Often – overexcitation, pathological feeling of fear, obsessive thoughts, disruption of normal sleep.
Nervous system
Often – headache.
Rarely - tremor of the limbs, sensory disturbances, crawling sensations on the skin, noise and ringing in the ears, attacks of dizziness, gait disturbances, fainting.
The cardiovascular system
Rarely - the development of arrhythmia, tachycardia, a feeling of interruptions in the functioning of the heart, discomfort and pain in the heart area, an increase or decrease in blood pressure, the development of a hypertensive crisis.
Respiratory system
Often – infectious lesions of the respiratory tract.
Rarely – development of dyspnea, apnea.
Digestive organs
Often – dyspeptic manifestations.
Rarely - a feeling of a metallic taste in the mouth, loss of appetite, vomiting, nausea, bloating, diarrhea, dry mouth, increased salivation, abdominal pain.
Musculoskeletal system
Rarely - dorsalgia, muscle spasms and muscle weakness.
Excretory system
Rarely – increased frequency of urination.
General reactions of the body
Increased fatigue, increased temperature and feeling of chills, asthenic manifestations, swelling of soft tissues, feeling of cold or heat, increased sweating.
To avoid the development of side effects during treatment with Mildronate, you must strictly follow the doctor’s instructions.
If a patient taking Mildronate is prescribed certain laboratory and instrumental diagnostic methods, possible changes in the results should be taken into account:
- dyslipidemic manifestations;
- increased concentration of CRP in the blood;
- increased levels of eosinophils in the blood;
- sinus tachycardia on ECG.
Analogs
Analogs of Mildronate: Vasopro, Vazonat, Metamax, Metonat, Trizipine, Mildrakor, Mildrocard, Cardionat, Melfor, Idrinol, Riboxin, Meldonium.
Mildronate is often compared to Cardionate. The fact is that these drugs are based on one active substance, so both drugs have a similar mechanism of action. But they differ in price and release form. You can find Cardionate in the pharmacy only in the form of 250 mg capsules and 500 mg/5 ml injection solution.
Analogs
Also, in the list of the most common replacements for Mildronate tablets with similar drugs is Riboxin. The drug is a means of metabolic action, that is, it participates in biochemical reactions. It has a positive effect on metabolic processes in the heart muscle and improves coronary circulation. Mildronate has a similar effect, but is not itself involved in the synthesis of other substances. Therefore, the effect of Mildronate lasts longer, and the body requires much less of it than Riboxin. As a result, it turns out that the combination of drugs will be optimal.
Important! The decision on combination therapy or replacement of Mildronate with an analogue is made by the attending physician.
Contraindications for use in athletes
The benefits of the drug for athletes (professionals and amateurs) are obvious. When taking the medicine:
- The statistical and dynamic activity of a person increases. The body's capabilities are expanded. Results and indicators are getting better.
- By stimulating the penetration of nutrients into the muscles (including the heart), the drug helps to increase the effectiveness of athletes’ training, as well as relieve fatigue.
- The heart begins to work better, the athlete becomes more resilient.
- Restoration of the body's energy reserves occurs in a shorter period of time, due to the active removal of metabolic products.
Mildronate can significantly improve athletic performance, but is prohibited for use by the World Anti-Doping Agency
At a stretch, the drug can be classified as a doping, since it helps to increase the body’s abilities, rather than physical performance.
However, the World Anti-Doping Agency (WADA) has recognized Mildrotan as a doping agent since 2016, which made its use impossible for professional athletes.
Indications
All dosage forms (capsules, tablets, injections) of Mildronate are intended for the treatment of adult patients. A doctor may prescribe the drug in the following cases:
- IHD (in combination with other drugs and treatment methods);
- peripheral arterial disease;
- reduced performance;
- encephalopathy;
- physical overload (including in sports);
- postoperative period (to speed up the recovery of the body);
- chronic heart failure;
- cardialgia (pain in the left side of the chest) caused by dishormonal myocardiopathy;
- bronchial asthma;
- COPD;
- alcohol withdrawal (as an addition to specific therapy);
- stroke.
The drug may be prescribed for reduced performance
Additional indications for the use of drug injections:
- hemorrhage into the vitreous cavity of the eye (hemophthalmos);
- hemorrhage in the retina of the eye;
- thrombosis and occlusion of the central retinal vein or its branches;
- retinopathy of various origins (for example, hemorrhagic or diabetic).
Prohibited for pregnant women, nursing mothers and children
Meldonium is contraindicated for use during pregnancy, since there is no data on its effect on the woman’s body and the development of the fetus/embryo (such clinical studies are contraindicated for ethical reasons). And existing data from animal studies are insufficient to draw a conclusion about the effect of the drug on the fetus.
Mildronate passes into breast milk. Unfortunately, it is unknown how the drug affects the body of a newborn child. Therefore, its use by the mother during lactation is contraindicated.
There are no data on the use of the drug in children (under 18 years of age). Therefore, the use of meldonium in this age category of patients is contraindicated.
The product is prohibited for use in pregnant women and nursing mothers
Overdose and its consequences
Until now, there have been no reports of cases of overdose with Mildronate, since the drug is low-toxic and does not cause severe side effects. In case of poisoning with the drug, the following symptoms are possible: decreased blood pressure, headache, palpitations, general weakness.
In cases of severe poisoning, liver and kidney dysfunction may occur. Treatment of poisoning is symptomatic. Hemodialysis is ineffective due to the high degree of binding of the drug to plasma proteins.
Reviews
Sergey, 28 years old, Bryansk I couldn’t get rid of excess weight. It was impossible to play sports regularly. A few light workouts a week in the form of jogging was all I was capable of due to my laziness and obesity. A friend, a professional athlete, advised me to drink Mildronate. Activity has increased. I started running every morning and regularly visiting the gym. As a result, I lost 15 kg, got rid of my complexes, and found my girlfriend. Thanks to the drug.
Elena, 32 years old, Belgorod I try to keep myself in good shape. Not professionally, but I do sports, and at the same time I drink Mildronate. After taking the drug again, I went to my dad’s birthday party and drank a small amount of alcohol. The heart began to beat very quickly, the parents got scared and called an ambulance. The doctors explained that these were side effects of the drug used.
Vladislav Petrovich, general surgeon, Samara I prescribe this medicine in combination with other drugs to patients in the postoperative period, after consulting with a cardiologist. Contraindications to the use of Mildronate are minimal, and the duration of rehabilitation is significantly reduced.
How to take Mildronate
The treatment regimen should be determined by the attending physician, taking into account the severity of the disease and the patient’s medical history. Self-medication can only worsen the condition.
Before taking Mildronate, be sure to consult your doctor!
The drug is taken at 500-1000 mg per day. The appointment can be one-time or divided into appointments throughout the day. It is worth noting that Mildronate can provoke overexcitation, so it is recommended to take it in the first half of the day and no later than 17:00. As a rule, the therapeutic course lasts from 4 to 6 weeks.
The tablets should be taken whole, without chewing or crushing in any other way. Injections are given intravenously, parabulbarly or intramuscularly. Moreover, Mildronate is administered intravenously separately from other medications. It does not need to be diluted with sodium solution. It is not recommended to administer Mildronate intramuscularly, as pain may occur or an allergic reaction may develop.
| Disease | Course duration | Dosage |
| Ischemia (angina pectoris, myocardial infarction), chronic heart failure | 4-6 weeks | As part of complex therapy 0.5-1 g 1-2 times/day |
| Cardialgia developing against the background of dyshormonal myocardial dystrophy | 12 days | 250 mg 2 times/day |
| Cerebrovascular insufficiency | 4-6 weeks | As part of complex therapy 0.5-1 g 1-2 times/day |
| Chronic cerebrovascular accidents | 2-3 times a year for 4-6 weeks | As part of complex therapy 500 mg/day |
| Physical and mental stress and decreased performance | 10-14 days | 500 mg 2 times/day |
| Athletes before training | 14-21 days during the preparatory period and 10-14 days during competitions | 0.5-1 g 2 times/day |
| Chronic alcoholism and withdrawal syndrome | 7-10 days | As part of complex therapy 0.5 g 4 times a day |
| After a heart attack | 4-5 weeks | On the first day, 0.5-1 g of solution is administered intravenously. Then the patient is transferred to tablets - 250 mg 2 times / day |
| Bronchial asthma | 3 weeks | As part of complex therapy 1 time/day |
| Vascular pathologies of the fundus | 10 days | Retrobulbar or subconjunctival 0.5 ml |
| Coronary syndrome | As prescribed by a doctor | IV 0.5-1 g solution 1 time/day. |
It is worth noting that Mildronate is combined with the following medications:
- diuretics;
- bronchodilators;
- antiplatelet agents;
- antiarrhythmic drugs;
- antianginal drugs.
In some cases, Mildronate, due to increased action and the possible development of moderate tachycardia and a decrease in blood pressure, should use the following medications with caution:
- cardiac glycosides;
- beta blockers;
- drugs that lower blood pressure.
As for taking Mildronate in combination with alcohol, in general there is no direct prohibition. However, the combination of the drug and alcoholic beverages can provoke:
- tachycardia;
- pronounced allergic reactions;
- sharp fluctuations in blood pressure;
- dyspeptic symptoms.
The increased risk of various types of complications and the likelihood of relapse of the disease indicate that alcohol should be excluded for the entire period of treatment.