Instructions for use NORVASC
- 1. Amlodipine dilates peripheral arterioles and, thus, reduces peripheral vascular resistance (afterload), which requires the work of the heart to overcome. Since heart rate does not change, reducing the workload on the heart leads to a decrease in energy consumption and oxygen demand.
2. The mechanism of action of amlodipine probably also includes dilation of the main coronary arteries and coronary arterioles in both unchanged and ischemic areas of the myocardium. This dilatation increases the supply of oxygen to the myocardium in patients with vasospastic angina (Prinzmetal's angina or variant angina) and prevents the development of coronary vasoconstriction caused by smoking.
In patients with arterial hypertension, a single daily dose of amlodipine provides a clinically significant reduction in blood pressure over 24 hours, both in the supine and standing positions. Due to its slow onset of action, amlodipine does not cause acute arterial hypotension.
In patients with angina pectoris, a single daily dose of amlodipine increases the time of physical activity, delays the development of an angina attack and ST segment depression (by 1 mm) during exercise, reduces the frequency of angina attacks and the consumption of nitroglycerin tablets.
Amlodipine does not have a negative effect on metabolism and the lipid profile of blood plasma, so it is suitable for the treatment of patients with bronchial asthma, diabetes mellitus and gout.
Use in patients with coronary artery disease
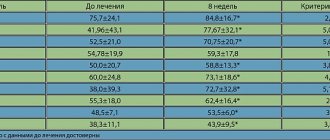
The effects of amlodipine on cardiovascular morbidity and mortality, progression of coronary atherosclerosis and the course of carotid atherosclerosis were studied in the Prospective Randomized evaluation of the Vascular Effects of NORVASC - PREVENT. This multicenter, randomized, double-blind, placebo-controlled study followed 825 patients with angiographically confirmed coronary atherosclerosis for 3 years. The study included patients who had undergone myocardial infarction (45%), percutaneous transluminal coronary angioplasty (PCA) (42%), or suffered from angina pectoris (69%). The severity of IHD varied from damage to one vessel (45% of patients) to stenosis of 3 or more arteries (21%). Patients with uncontrolled hypertension (diastolic blood pressure > 95 mmHg) were excluded from the study. The presence of major cardiovascular events was confirmed by a blinded endpoint committee. Although the incidence of progression of coronary atherosclerosis did not change, amlodipine prevented progressive carotid intima-media thickening. In patients receiving amlodipine, there was a significant reduction (by 31%) in the total incidence of death from cardiovascular causes, myocardial infarction, stroke, TLP, coronary artery bypass grafting, hospitalization for unstable angina, and progression of congestive heart failure. In addition, in patients in the amlodipine group, the frequency of interventions aimed at restoring coronary blood flow (TLP and coronary artery bypass grafting) decreased significantly (by 42%). When treated with amlodipine, the incidence of hospitalization for unstable angina was lower (by 33%) than in the placebo group.
Use in patients with heart failure
Hemodynamic studies and controlled clinical studies using exercise testing have shown that amlodipine does not cause deterioration in patients with NYHA functional class II-IV heart failure, as evidenced by the study of exercise tolerance, left ventricular ejection fraction and clinical symptoms.
In a placebo-controlled study (PRAISE), which included patients with NYHA functional class III-IV heart failure treated with digoxin, diuretics and ACE inhibitors, amlodipine did not increase the risk of death or the total risk of complications and death.
In a long-term placebo-controlled study (PRAISE-2) in patients with NYHA functional class III-IV heart failure without clinical or objective signs of coronary heart disease who received ACE inhibitors, cardiac glycosides and diuretics in constant doses, amlodipine had no effect on overall and cardiovascular mortality. In the same population, amlodipine was associated with an increased incidence of pulmonary edema, although the incidence of progression of heart failure was not significantly different from placebo.
Norvasc®
Amlodipine can be safely used for the treatment of arterial hypertension together with thiazide diuretics, alpha-blockers, beta-blockers or ACE inhibitors.
In patients with stable angina, amlodipine can be combined with other antianginal agents, for example, long- or short-acting nitrates, beta-blockers.
Unlike other BMCCs, no clinically significant interaction of amlodipine (III generation BMCCs) was found when used together with non-steroidal anti-inflammatory drugs (NSAIDs), including indomethacin.
It is possible to enhance the antianginal and hypotensive effect of BMCC when used together with thiazide and loop diuretics, ACE inhibitors, beta-blockers and nitrates, as well as to enhance their hypotensive effect when used together with alpha1-blockers, antipsychotics.
Although negative inotropic effects have generally not been observed in amlodipine studies, some CBMCs may enhance the negative inotropic effects of antiarrhythmic drugs that cause QT prolongation (eg, amiodarone and quinidine).
Amlodipine can also be safely used concomitantly with antibiotics and oral hypoglycemic agents.
A single dose of 100 mg of sildenafil in patients with essential hypertension does not affect the pharmacokinetic parameters of amlodipine.
Repeated use of amlodipine at a dose of 10 mg and atorvastatin at a dose of 80 mg is not accompanied by significant changes in the pharmacokinetics of atorvastatin.
Simvastatin
: Simultaneous repeated use of amlodipine at a dose of 10 mg and simvastatin at a dose of 80 mg leads to an increase in simvastatin exposure by 77%. In such cases, the dose of simvastatin should be limited to 20 mg.
Ethanol
(drinks containing alcohol):
amlodipine with single and repeated use in a dose of 10 mg does not affect the pharmacokinetics of ethanol.
Antivirals (ritonavir):
increases plasma concentrations of BMCC, including amlodipine.
Neuroleptics and isoflurane:
enhancing the hypotensive effect of dihydropyridine derivatives.
Calcium preparations
may reduce the effect of BMCC.
When combined with BMCC and lithium preparations
(no data available for amlodipine), possibly increasing the manifestation of their neurotoxicity (nausea, vomiting, diarrhea, ataxia, tremor, tinnitus).
Studies of the simultaneous use of amlodipine and cyclosporine in healthy volunteers and all groups of patients, with the exception of patients after kidney transplantation, have not been conducted.
Various studies of the interaction of amlodipine with cyclosporine in patients after kidney transplantation show that the use of this combination may not lead to any effect, or increase the minimum concentration of cyclosporine to varying degrees, up to 40%. These data should be taken into account and cyclosporine concentrations should be monitored in this group of patients when cyclosporine and amlodipine are used concomitantly.
Does not affect serum digoxin
and its renal clearance.
Does not significantly affect the action of warfarin
(prothrombin time).
Cimetidine
does not affect the pharmacokinetics of amlodipine.
in vitro studies
amlodipine does not affect the plasma protein binding of
digoxin
,
phenytoin
,
warfarin
and
indomethacin
.
Grapefruit juice:
A simultaneous single dose of 240 mg of grapefruit juice and 10 mg of amlodipine orally is not accompanied by a significant change in the pharmacokinetics of amlodipine. However, it is not recommended to use grapefruit juice and amlodipine at the same time, since genetic polymorphism of the CYP3A4 isoenzyme may increase the bioavailability of amlodipine and, as a result, enhance the hypotensive effect.
Aluminum- or magnesium-containing
antacids
: their single dose does not have a significant effect on the pharmacokinetics of amlodipine.
CYP3A4 isoenzyme inhibitors
: with simultaneous use of diltiazem at a dose of 180 mg and amlodipine at a dose of 5 mg in patients from 69 to 87 years of age with arterial hypertension. There is an increase in systemic exposure of amlodipine by 57%. Concomitant use of amlodipine and erythromycin in healthy volunteers (18 to 43 years of age) does not lead to significant changes in amlodipine exposure (increase in area under the concentration-time curve (AUC) by 22%). Although the clinical significance of these effects is unclear, they may be more pronounced in older patients.
Potent inhibitors of the CYP3A4 isoenzyme (for example, ketoconazole, itraconazole) may increase the plasma concentration of amlodipine to a greater extent than diltiazem. Amlodipine and inhibitors of the CYP3A4 isoenzyme should be used with caution.
Clarithromycin:
CYP3A4 isoenzyme inhibitor. Patients taking clarithromycin and amlodipine at the same time have an increased risk of low blood pressure. Patients taking this combination are advised to be under close medical supervision.
Inducers of the CYP3A4 isoenzyme
: There is no data on the effect of inducers of the CYP3A4 isoenzyme on the pharmacokinetics of amlodipine. Blood pressure should be carefully monitored during concomitant use of amlodipine and inducers of the CYP3A4 isoenzyme.
Tacrolimus
: When used simultaneously with amlodipine, there is a risk of increasing the concentration of tacrolimus in the blood plasma. To avoid toxicity of tacrolimus when used concomitantly with amlodipine, the concentration of tacrolimus in the blood plasma of patients should be monitored and the dose of tacrolimus should be adjusted if necessary.
Mammalian target of rapamycin (mTOR) inhibitors
mTOR inhibitors such as sirolimus, temsirolimus and everolimus are substrates of the CYP3A isoenzyme. Amlodipine is a weak inhibitor of the CYP3A isoenzyme. When used concomitantly with mTOR inhibitors, amlodipine may increase their exposure.
Side effects of the drug Norvasc
In placebo-controlled clinical trials involving patients with hypertension (arterial hypertension) and angina pectoris, the most commonly reported symptoms were headache, peripheral edema, fatigue, drowsiness, nausea, abdominal pain, flushing, palpitations and dizziness. In these clinical studies, no clinically significant changes in laboratory parameters due to Norvasc were noted. Significantly less often, with the widespread use of Norvasc, side effects such as alopecia, constipation, arthralgia, asthenia, back pain, dyspepsia, shortness of breath, gum hyperplasia, gynecomastia, hyperglycemia, impotence, increased frequency of urination, leukopenia, general malaise, mood changes, sensation were observed. dry mouth, convulsions, myalgia, peripheral neuropathy, pancreatitis, sweating, fainting, thrombocytopenia, vasculitis. In most cases, the causal relationship was unreliable. Allergic reactions have been reported rarely, including pruritus, rash, angioedema and erythema multiforme. Very rarely, when using the drug, hepatitis, jaundice and increased activity of liver enzymes (usually due to cholestasis) were observed. In most of these cases, the causal relationship was not reliable. As with the use of other calcium ion antagonists, isolated cases of myocardial infarction, arrhythmias (including ventricular tachycardia and atrial fibrillation), as well as chest pain have been described with the use of Norvasc.
Norvasc drug overdose, symptoms and treatment
Experience in treating Norvasc overdose in humans is limited. In some cases, gastric lavage is advisable. Existing data suggest that significant overdose may lead to excessive peripheral vasodilation with a subsequent marked and possibly prolonged decrease in blood pressure. Clinically significant arterial hypotension caused by an overdose of Norvasc requires active measures aimed at maintaining the function of the cardiovascular system, including monitoring of heart and lung parameters, elevated position of the lower extremities, control of circulating blood volume and diuresis. To restore vascular tone and normalize blood pressure, vasoconstrictor drugs can be used if there are no contraindications to their use. In order to eliminate the consequences of calcium channel blockade, intravenous administration of calcium gluconate solution is indicated. Since amlodipine is highly bound to plasma proteins, dialysis is ineffective.
Special instructions for the use of Norvasc
The half-life of Norvasc increases in patients with impaired liver function, however, recommendations regarding the dosage regimen of the drug in this group of patients have not been developed, for this reason the drug should be used in them with caution. The recommended dose for patients with liver failure is 2.5 mg. In patients with renal failure, Norvasc is used in normal doses. Changes in plasma concentrations of amlodipine do not correlate with the severity of renal failure. Amlodipine is not eliminated by dialysis. Elderly patients tolerate the drug well; The recommended dose for them is 2.5 mg/day. The safety of using Norvasc during pregnancy and lactation has not been established. The drug can be used during pregnancy only if there is no safer alternative, and the risk caused by the disease itself outweighs the possible negative consequences of using the drug for the mother and fetus. The effectiveness and safety of Norvasc in children has not been studied. It is unlikely that Norvasc can have a negative effect on the ability to drive vehicles or operate potentially dangerous machinery.
Possibilities of the calcium antagonist amlodipine (Norvasc®) in the treatment of cardiovascular diseases
Today it is even difficult to imagine that just 60 years ago, doctors did not pay attention to high blood pressure (BP), and treatment was prescribed, as a rule, only when the symptoms of the disease were significant. In the chapter on arterial hypertension (AH), in the first edition of the Manual of Internal Medicine, edited by Harrison (1950), it was stated that “specific drug therapy with veratrine, tetraethylammonium and other drugs is too dangerous and rarely effective to be used for routine treatment." According to WHO, 15–20% of the adult population suffer from hypertension, which is one of the causes of mortality from cardiovascular diseases, a cause of disability and associated costs. It is well known that hypertension is one of the most important risk factors for the development of coronary heart disease (CHD) and cerebral vascular damage. End of the 20th century was marked by intensive development of ideas about the pathogenetic basis of hypertension, a critical revision of many causes, mechanisms and treatment of this disease, the creation of new classes of antihypertensive drugs and the formation of a fundamentally new approach to assessing the effectiveness of treatment - evidence-based medicine. One of the most difficult tasks for a clinician has become the choice of the optimal drug for the treatment of a patient with hypertension. The difficulty was that, in the absence of specific indications, there are several classes of effective antihypertensive drugs, any of which can be used in this situation. Numerous completed randomized studies have not found significant benefits for any of the 6 classes of antihypertensive drugs in terms of the severity of blood pressure reduction. It is clear that the attention paid to the ability of drugs to reduce cardiovascular morbidity and mortality while maintaining a good quality of life. The WHO/IOAG guidelines (1999) expected that the most important differences between classes of antihypertensive drugs (diuretics, β-blockers, calcium antagonists (CA), α-blockers, angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers) were the presence or absence of evidence obtained from randomized clinical trials that examined the effect of these drugs on morbidity and mortality in hypertension [27]. AKs have been used in cardiology for more than 35 years. Their widespread use in clinical practice is facilitated by their high anti-ischemic and antianginal efficacy, as well as good tolerability, established in large clinical studies [1,6,14]. The main point of application of drugs of this group at the cellular level are slow calcium channels, through which calcium ions pass into the smooth muscle cells of blood vessels and the heart. In the presence of calcium ions, actin and myosin interact, ensuring contractility of the myocardium and smooth muscle cells. In addition, calcium channels are involved in the generation of pacemaker activity of sinus node cells and conduction of impulses through the atrioventricular node. The mechanism of action of AKs is as follows: 1) a decrease in afterload on the heart due to their peripheral vasodilating effect and a decrease in systemic vascular resistance; 2) direct negative inotropic effect on the myocardium (verapamil and diltiazem); 3) improvement of myocardial perfusion during ischemia due to relief and prevention of spasm of the coronary arteries and reduction of their resistance [2,10,15]. It has been established that the vasodilating effect caused by AA occurs not only through a direct effect on the smooth muscles of the vascular wall, but also indirectly, through potentiation of the release of nitric oxide from the vascular endothelium. This phenomenon was found in most dihydropyridines and isradipine, and to a lesser extent in nifedipine and non-hydropyridine drugs. The first generation (generation) of AK are conventional tablets: verapamil, diltiazem, nifedipine, felodipine, isradipine, nicardipine, nitrendipine. Second generation AK (modified release): verapamil SR, diltiazem CD, nifedipine XL, felodipine ER, isradipine CR. Third generation AK (long-acting drugs): amlodipine, lacidipine, lercanidipine. 2nd and 3rd generation AK drugs are more active and specific for certain organs and tissues, and have a longer effect (amlodipine, nisoldipine, felodipine, etc.). The positive properties of these AKs include: greater specificity to organs and vascular areas, suitability for prophylactic use, weakening of many side effects characteristic of 1st generation drugs, as well as the emergence of drugs with new additional properties, for example, antiplatelet activity against platelets ( trapidil). 3rd generation drugs (amlodipine) are increasingly used in the clinic. These drugs are characterized by a long-term antihypertensive effect with minimal effect on myocardial contractile function and intracardiac conduction [4,5,8,11]. The use of amlodipine in hypertension Vasodilation while taking amlodipine is due not only to the blockade of calcium channels, but also to stimulation of the release of nitric oxide (NO) by endothelial cells, which is a powerful natural vasodilator, as well as increased release of bradykinin. The hypotensive effect of amlodipine develops gradually, has a dose-dependent effect, and does not cause a serious reflex increase in heart rate (HR); addiction does not develop to the effect of the drug with long-term use [1]. In a large prospective comparative clinical study ASCOT-BPLA [12], which was conducted in 19,257 patients with hypertension (BP≥160/100 mmHg) aged 40 to 79 years at average risk (elevated blood pressure≥140/90 mmHg) Hg and the presence of at least three additional risk factors), the advantage of an antihypertensive therapy regimen based on the AC amlodipine 5–10 mg with the addition of the ACE inhibitor perindopril 4–8 mg was shown compared with a regimen based on the b-blocker atenolol 50–100 mg with the addition of the thiazide diuretic bendroflumethiazide in reducing the risk of CVD and diabetes mellitus. The duration of observation was 5.5 years. The study was completed ahead of schedule due to the obvious advantage of therapy with amlodipine and perindopril in terms of preventing not only major CVDs, but also a significant reduction in the risk of death from all causes. The ACCOMPLISH study [3,7] included 11,506 patients with hypertension at high risk of developing CVD complications with a history of complications of coronary artery disease, including myocardial infarction (MI), myocardial revascularization, as well as previous stroke, renal dysfunction, peripheral arterial disease, left ventricular (LV) myocardial hypertrophy or diabetes mellitus. It turned out that the combined use of benazepril and amlodipine is more effective than the combined use of benazepril and hydrochlorothiazide in reducing the incidence of CVD in patients with hypertension at high risk of developing such complications. The long-term (mean follow-up period 4.4 years) multicenter comparative prospective study TOMHS [12], sponsored by the US National Institutes of Health, compared the effectiveness of long-term therapy with various drugs (amlodipine, enalapril, chlorthalidone, doxazosin, acebutolol) in 903 patients with mild hypertension ( diastolic blood pressure (DBP) from 90 to 99 mmHg) for 4 years. Treatment was considered effective if DBP was less than 90 mmHg. The research results showed that in the amlodipine group, treatment was more effective in 83% of patients, in the enalapril group - in 68%, in the chlorthalidone group - 68%, placebo - 59%. The double-blind, randomized study ELVERA [24-25] noted a significant reduction in LV hypertrophy in 166 elderly patients (60–75 years old) with hypertension and proven LV hypertrophy while taking 5–10 mg of amlodipine for 2 years, comparable to the effect of taking 10–10 mg of amlodipine. 20 mg of the ACE inhibitor lisinopril. The data obtained on the reverse development of myocardial damage associated with LV hypertrophy (the target organ of hypertension) may explain the beneficial effect of amlodipine on the risk of cardiovascular complications (CVD). The CAMELOT study [21] compared the effects of the AC amlodipine and the ACE inhibitor enalapril under placebo control in patients with coronary artery disease with normal blood pressure. The study was a double-blind, randomized, multicenter, 24-month study conducted in 1991 patients with angiographically proven CAD and diastolic blood pressure less than 100 mmHg. 673 people were included in the enalapril group (20 mg/day), 663 people in the amlodipine group (10 mg/day), and 655 patients in the placebo group. The main parameter of drug efficacy was the incidence of cardiovascular events when taking amlodipine compared with placebo, which included death due to a given event, non-fatal myocardial infarction, successful resuscitation of cardiovascular events during cardiac arrest, coronary revascularization, hospitalization due to angina, hospitalization due to congestive heart failure. heart failure, fatal and non-fatal stroke (transient cerebrovascular accident) and newly diagnosed peripheral vascular disease. In 274 patients, the progression of atherosclerosis (percentage change in the volume of atherosclerotic plaques) was assessed using intravascular ultrasound (IVUS). In the generalized sample of patients, the average blood pressure level was 120/78 mm Hg. The results of the study were as follows: in the placebo group after 24 months. Blood pressure increased by 0.7/0.6 mm Hg, and in the groups using amlodipine and enalapril blood pressure decreased by 4.8/2.5 and 4.9/2.4 mm Hg. respectively (p<0.001 for both groups compared with placebo). CV events occurred in 151 (23.1%) patients in the placebo group, 110 (16.6%) receiving amlodipine (hazard ratio (HR) = 0.69; 95% confidence interval (CI) - 0.54 - 0.88; p=0.003), and in 136 (20.2%) patients receiving enalapril (HR=0.85; 95% CI - 0.67–1.07; p=0.16). The main parameter, the difference in the incidence of cardiovascular events between enalapril and amlodipine, turned out to be statistically insignificant. IVUS revealed a trend toward decreased progression of atherosclerosis in the amlodipine group compared with the placebo group (p=0.12), with significantly less progression in the subgroup with above-average systolic blood pressure (p=0.02). Compared with baseline, IVUS showed an increase in plaques in the placebo group (p < 0.001), a trend towards progression of atherosclerosis in the enalapril group (p = 0.08) and no progression in the amlodipine group (p = 0.031). In the amlodipine group, the correlation indicator (r) between the decrease in blood pressure and the progression of atherosclerosis was 0.19 (p = 0.07). When taking amlodipine, the need for revascularization decreased by 27.4%, the frequency of hospitalizations for angina pectoris (by 42.2%) and the frequency of non-fatal myocardial infarction (by 26%), as well as the incidence of stroke or transient cerebrovascular insufficiency (by 50.4%). %). It is important to note that improvement in clinical outcomes was observed with adequate lipid-lowering therapy (lipoprotein levels). Improvement in clinical outcomes was observed against the background of adequate lipid-lowering therapy (low-density lipoprotein level was 100 mg/dl (2.59 mmol/l) and concomitant therapy with acetylsalicylic acid and b-blockers. It was concluded that the administration of amlodipine to patients with coronary artery disease and normal blood pressure led to to a decrease in the incidence of cardiovascular events. When taking enalapril, a similar, but less pronounced and not statistically significant effect was observed. IVUS demonstrated a slowdown in the progression of atherosclerotic vascular lesions when taking amlodipine. The place of amlodipine in the treatment of coronary heart disease Treatment of patients with coronary artery disease is aimed at preventing death, myocardial infarction, and reducing symptoms angina pectoris and the development of myocardial ischemia. By dilating peripheral vessels, amlodipine reduces the afterload of the heart. Due to the fact that the drug does not cause reflex tachycardia (since the sympathetic-adrenal system is not activated), energy consumption and the need for oxygen of the myocardium are reduced. The drug expands the coronary arteries and enhances the supply of oxygen to the myocardium. The antianginal effect (reducing the duration of angina attacks, the daily requirement for nitroglycerin), increasing exercise tolerance, improving systolic and diastolic heart function, in the absence of a depressing effect on the sinus and atrioventricular node and other elements of the conduction system of the heart, makes the drug one of the first places in the treatment of angina pectoris [3,7,9]. Amlodipine dilates the main coronary arteries and arterioles (including in ischemic areas of the myocardium) and prevents the development of spasm of the coronary arteries. Thus, the drug improves the supply of oxygen to the myocardium while reducing the need for it, which makes it possible to use it in the treatment of angina pectoris. The CAPE study [13,18] showed that amlodipine, added to the basic therapy of patients with angina, prevents anginal attacks and silent myocardial ischemia within 24 hours. The international double-blind placebo-controlled CAPE II study [13], which lasted 8 weeks, 315 patients suffering from stable angina pectoris and receiving amlodipine (Norvasc®) at a dose of 5–10 mg/day were included. or placebo. 65% of patients in this study were already taking b-blockers before prescribing amlodipine (Norvasc®). It has been shown that taking amlodipine (Norvasca®) once a day. was accompanied by a decrease in the frequency of angina attacks over 24 hours. When treated with amlodipine (Norvasc), an increase in physical activity was noted by a significantly larger number of patients (75%) than when taking placebo (59%, p = 0.003). The drug significantly reduced the frequency of episodes of ischemic depression of the ST segment according to ECG-Holter monitoring, as well as the frequency of painful attacks and the need for the use of short-acting nitrates. Due to adverse events, only 2.0% of patients stopped treatment with amlodipine (Norvasc), compared to 4.4% when taking placebo. In the double-blind, placebo-controlled CAPARES study [16], amlodipine was administered at a dose of 10 mg/day in 585 patients who underwent coronary angioplasty. prescribed 2 weeks in advance. before and during 4 months. after the intervention. Although amlodipine therapy did not affect the development of restenosis after coronary angioplasty (28.1% restenosis in the amlodipine group and 28.4% in the placebo group), it reduced the total risk of adverse outcomes by 35%, including death, myocardial infarction, aortic coronary artery disease. bypass surgery or repeat angioplasty. Thus, in patients with stenosing coronary atherosclerosis, amlodipine showed better clinical results even with a short duration of treatment (4 months), which does not exclude the presence of the drug not only antianginal, anti-ischemic, but also anti-remodeling and anti-atherogenic effects. In the PREVENT study [23] (a multicenter, prospective, randomized, double-blind, placebo-controlled study), the main purpose of which was to evaluate the effect of amlodipine (treatment duration of 3 years) on the progression of atherosclerosis in the carotid and coronary arteries in 825 patients with coronary artery disease. It turned out that amlodipine significantly slowed down the development of the atherosclerotic process in the carotid arteries compared to the placebo group. The effect of the drug on the dynamics of coronary artery lesions was unreliable according to quantitative coronary angiography. When comparing clinical results such indicators as overall mortality, fatal and non-fatal myocardial infarction, the total number of all complications of coronary artery disease was 31% lower (p = 0.01), which proves the favorable clinical effects of amlodipine. There was a decrease in the number of hospitalizations due to destabilization of angina pectoris and chronic heart failure (61 in the amlodipine group and 88 in the placebo group). Also, with the use of amlodipine, a decrease in the number of myocardial revascularization operations was observed (53 compared with 85 in the placebo group), regardless of the use of b-blockers, nitrates or lipid-lowering therapy. The frequency of anginal attacks decreased from 85 to 60. Amlodipine in heart failure (HF) A beneficial effect of amlodipine was obtained in patients with chronic HF. The double-blind, placebo-controlled PRAISE study [22] demonstrated the safety and effectiveness of amlodipine in patients with hypertension in combination with severe heart failure. The duration of the study ranged from 6 to 33 months. (average 13.6 months). It included 1153 patients (ejection fraction less than 30%). In 732 of them, HF was combined with coronary artery disease and in 421 with non-ischemic cardiomyopathy. All patients were randomized to groups receiving placebo or amlodipine at an initial dose of 5 mg/day. within 2 weeks. in addition to standard treatment for heart failure; then the dose was increased (if well tolerated) to 10 mg/day. The addition of amlodipine resulted in a nonsignificant reduction in mortality (relative risk 0.84, 95% CI 0.69–1.02). An increase in the survival rate of patients with non-ischemic dilated cardiopathy was noted. The favorable effect is explained by the fact that the antihypertensive effect of the drug occurs slowly, it does not stimulate either sympathetic or renin -angiotensin -aldosterone systems, does not have a negative foreign effect, but slows down the progression of myocardial hypertrophy. The influence of amlodipine on the progression of vessel atherosclerosis due to the presence of a number of reports about the possible favorable effect of calcium antagonists on the state of the vascular endothelium conducted a multicenter prospective, randomized double blind, placebo -controlled study of Prevent [23]. In the course of this study, a change in the degree of atherosclerotic damage to the coronary arteries and the thickness of the intimate -medial layer of carotid arteries against the background of the purpose of amlodipine was evaluated. The study was conducted on 825 patients with the presence of coronary emergency Coronarography. Patients received amlodipine at a dose of 5 mg, which, if well tolerated, was increased to 10 mg. As a result of the study, there was no reliable difference in both studied groups - active treatment and control - in relation to the progression of atherosclerotic stenization of coronary vessels. On the contrary, there was a pronounced effect of amlodipine on the progression of atherosclerosis in the carotid arteries, detected using ultrasonography. At the same time, in the amlodipine group there was a regression of the intimate -medial layer by 0.046 mm, and in the control group there was a thickening of 0.011 mm. Currently, a correlation of the degree of thickening of the intimate -medial layer of carbon arteries with the frequency of development and brain stroke has been proven. The Prevent [23] study also shows good tolerance of the drug, which was previously noted by other researchers, while the frequency of adverse reactions was comparable to the placebo group (79% for amlodipine and 83% for placebo). The additional purpose of angiotensin -converting enzyme inhibitors in the amlodipine group was two times less common than in the placebo group. The undoubted advantage of amlodipine is a wide range of pharmacological effects aimed at eliminating the manifestations of coronary failure - anti -angal, anti -thelays, anti -ischemic. Therapy with this drug and on the course of atherosclerosis affects favorably. Amlodipine has greater selectivity in relation to coronary and brain vessels, is practically deprived of a inotropic effect and effect on the function of the sinus node and atrio -antitrocular conductivity, which determines its advantage over other drugs in clinical practice. An advantageous difference between amlodipine and earlier calcium antagonists is its lack of influence on heart rate, an increase in which during physical activity is one of the triggers for ischemia. In addition, the drug is well tolerated by patients. Calcium channel blockers can be used for vasospastic angina pectoris, accompanying obstructive pulmonary diseases. An additional indication for the appointment of calcium antagonists is Raynaud's syndrome. Among the side effects is the possibility of developing edema of the legs and hyperemia, which is characteristic of all drugs of digidropyridine series. With a sudden cessation of amlodipin taking, no cancellation syndrome is observed. Thus, amlodipine is classified as the main drugs in the treatment of hypertension and angina pectoris. The drug is quite effective and well tolerated by patients. Calcium antagonists of dihydropyridine group of prolonged action are shown to patients with stable angina pectoris and elderly patients. Other favorable effects of AK in patients with coronary heart disease in combination with hypertension are: anti -adaterogenic, antiproroliferative, anti -agricultural. Amlodipine with a single use provides uniform control of blood pressure and is well tolerated by patients. For prolonged treatment of angina pectoris from dihydropyridine derivatives, it is recommended to use long -acting dosage forms. The subject of the study in recent years has become the effectiveness and safety of prolonged use of AK, as well as an assessment of their influence on the mortality and risk of various MTRs (them, impaired cerebral circulation, etc.) in patients with coronary heart disease and hypertension. Long -term, regular intake of modern drugs AK AK and stable angina pectoris reduces the mortality and risk of MTR (primarily brain strokes). Preparations have a proven anti -atherogenic effect. In middle -aged patients with soft hypertension and without severe concomitant diseases, the use of amlodipine as preparations of the 1st row is quite justified [20]. The issue of the therapeutic equivalence of Generica to the original drug is fundamental: for example, the ability to achieve the target level of blood pressure when used in the same doses as the original drug. In particular, comparative pharmacological studies of the original enalapril and generics showed that a daily dose of generic is necessary to achieve targeted blood pressure, 1.5–2 times and more exceeding the dose of the original drug [26]. Therefore, some doctors in the Russian Federation prescribe the patient a deliberately large dose of generic in comparison with the original drug. When choosing between a generic and an original drug, it is necessary to take into account the ratio of cost, efficiency and safety. Often the low price of the generic packaging is poured into the high cost of treatment: both due to high doses of the drug, and due to the cost of correcting side effects, and by adding other drugs [17]. The original drug amlodipine Norvask® Pfizer is one of the most commonly used cardiovascular drugs in the world. It has proven effectiveness with stable and vasospastic angina pectoris. The drug provided a pronounced additional antianginal effect if the therapy with the B - the Blocker turned out to be insufficient, which was confirmed by an increase in physical activity until the symptoms of angina pectoris appear [17]. In conclusion, it should be noted that doctors often face the problem of choosing a specific drug within the same pharmacological group. In the absence of comparative data on the frequency of complications and mortality, any judgment on the interchangeability of drugs is based on surrogate data on effectiveness. Since effective doses for a reliable reduction in the number of adverse events can only be determined during long -term, voluminous studies, new tests should be carried out to confirm this assumption. Unfortunately, small and short -term efficiency studies cannot serve as the basis for assessing safety when taking for a long time. In addition, there are currently few reliable data on generics in case of prolonged use. Many years of experience using original amlodipine in the clinical practice (Norvask®, Pfizer) in the Russian Federation and abroad allows us to talk about its leading place in pharmacotherapy AG and IBS and positively evaluate its prospects in cardiological practice. Literature 1. Belousov Yu.B., Gracean N.A. Clinical pharmacology of amlodipine (Norvaska). Univers Publ, 1998. 2. Diagnosis and treatment of stable angina pectoris. Russian recommendations. Designed by the NNC experts Committee (second review). Moscow, 2008. 40 p. 3. Dobrovolsky A.V. Calcium channel blockers are dihydropyridine derivatives in the treatment of diseases of the cardiovascular system. Russian honey Journal, 2005; t. 13, No. 27: 1850–1857 4. Karpov Yu.A. Stable coronary heart disease: new studies and prospects for the clinical use of calcium antagonists. Pharmacate, 2003; No. 12: 6–9. 5. Lupanov V.P. Calcium dihydropyridine antagonists in the treatment of patients with coronary heart disease and arterial hypertension. Russian honey Journal, 2005; t. 13, No. 19: 1282–1286. 6. Lupanov V.P. Calcium antagonists in the treatment of patients with chronic ischemic heart disease. Attending physician, 2006; No. 9: 76–82. 1-3. 7. Palunts O.B., Silina E.G. Amlodipine as a new look at calcium antagonists. Russian honey Journal, 2010; T.18, No. 9: 570–574. 8. Preobrazhensky V.V., Sidorenko B.A., Shabaeva E.N. Amlodipine is a third -generation calcium antagonist. Cardiology, 1998; No. 2: 66–73 9. Sidorenko B.A., Preobrazhensky D.V. Calcium antagonists. M.: AOZT Informatics, 1999. 176 p. 10. Syrkin A.L., Dobrovolsky A.V. Calcium channel blockers and their place in the treatment of arterial hypertension and coronary heart disease. Consilium Med, 2004; t. 6, No. 5: 272–276. 11. Feldsherova N.A., Semernin E.N. Amlodipine: review of clinical research. High -quality clinical practice 2002; 2:1–8. 12. Dahlof B., Sever PS, Poulter NR et al. for the ascot investigators. Prevention of Cardiovascular Events with Annypertensive Regime Adding Perindopril as Required Versus ATENOLOFLUMETHIAZIDE AS REQURE, In The ANGLO —Scandinavian Cardiac Outcomes Trial - Blood Pressure Lowering ARM (ASCOT - BPLA): A Multicentre Randomized Controlled Trial. Lancet, 2005; 366:895–906. 13. Detry JM Amlodipine and the Total Ischemic Burden: Circadian Anti - Ischemia Program in Europe (Cape) Trial. Methodology, Safety and Toleration. Cardiology, 1994; 85, SUPPL 2: 24–30. 14. Grossman Eimesserli FH Calcium Antagonists. Progress in Cardiovascular Dis 2004; vol. 47. No. 1: 34–57. 15. Guidelines on the Management of Stable Angina Pectoris - Executive Summary. The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology (Fox K. et al.) EUR Heart J, 2006; 27: 1341–1381. 16. Jordensen B., Simonsen S., Endresen K. et al. Restenosis and Clinical Outcome in Patents Treated with amlodipine after Anter Angioplasty: Results from the Cornary Angioplasty Amlodipine Restenosis Study (Capares). J Am Coll Cardiol, 2002; 35: 592–599. 17. Laroche ml, Merle L. Geneic and Brand - Name Drugs. Are Different Criteria 3sufficiently Taken Into Account Before Granting Market Authorism? Acta Clin Belg Suppl 2006; (1): 48–50. 18. Lichtlen PR, Fisher LD Analysis of Arrhythmias in the Circadian Antiischemia Program in Europe (Cape) Study. J Cardiovasc Pharmacol 1999 33(1) 135–9. 19. Mignini F, Tomassoni D, Traini E, et al. Single - Dose, Randomized, Crossover Bioequaleence Study of Amlodipine Maleate Versus Amlodipine Besylate in Healthy Volunteers. Clin Exp Hypertens 2007; 29: 539–52. 20. Neaton JD, Grimm Jr Rh, Prineas RJ, et al. Treatment of Mild Hypertension Study. Final Results. Jama 1993; 270: 713–24. 21. Nissen S., Tuzcu E., Libby P., et al. The effect of antihypertensive drugs on cardiovascular events in patients with coronary heart disease and normal blood pressure. Randomized controlled Camelot. Arterial hypertension, 2005; No. 2: 2–7. 22. Packer M. et al. Effect of Amlodipine on Morbidity and Mortaly in Severe Chronic Heart Failure. N English J Med 1996 335 1107–14. 23. Pitt B., Byington RP, Furberg CD Eal. Effect of amlodipine on the Progression of Atherosclerosis and the Ocureence of Clinical Events. Prevent Investigatiors. Circulation, 2000; 102: 1503–1510. 24. Terpstra WF, May JF, Smith AJ et al. Long -Term Effects of Amlodipine and Lisinopril On Left Ventriculam and Diastolic Function in Elderly, Previusly Untreed Hypertensive Patients: The ElVera Trial. J Hypertens, 2001; 19: 300–309. 25. Terpstra wf, May JF, Smith AJ et al. Effects of Amlodipine and Lisinopril On -Media Thickness in Previous Untreed, Elderly Hypertensive Patients (The Elvera Trial). J Hypertens, 2004; 22 (7): 1309–1316. 26. US Food and Drug Administration (FDA), Therapeutic Equivalence of Generic Drugs: Letter to Health Practitioners, January 1998. AVAIABLE AT: https://wwwww.fda.gov/cder/newww S/nightgenlett.htm. 27. US Food and Drug Administration (FDA), Center for Drug Evalving and Research (CDER), Orange Book - Approved Drug Products with Therapeutic Equivalence Evaluates, 28th EDN, 28TH EDN. 2008. Available at: https://www.fda.gov/ CDER/Orange/Obannual.pdf.
Indications for use of the drug Norvasc
Treatment of hypertension (arterial hypertension) (as a first-line drug as monotherapy or combination therapy in combination with thiazide diuretics, α- and β-adrenergic receptor blockers, ACE inhibitors). Treatment of coronary artery disease, namely stable angina and vasospastic angina (variant angina or Prinzmetal angina). Reducing the risk of the need for myocardial revascularization and hospitalization for angina pectoris in patients with coronary artery disease. The drug can be used in cases where the clinical picture suggests the presence of vasospasm or vasoconstriction, although their presence has not been confirmed. Used as monotherapy or in combination with other antianginal drugs.
Norvasc, 5 mg, tablets, 14 pcs.
Amlodipine can be safely used for the treatment of arterial hypertension together with thiazide diuretics, α-blockers, β-blockers or ACE inhibitors. In patients with stable angina, amlodipine can be combined with other antianginal agents, such as long-acting or short-acting nitrates, beta-blockers.
Unlike other CCBs, a clinically significant interaction with amlodipine (III generation CCB) was not detected when used together with NSAIDs, incl. and with indomethacin.
It is possible to enhance the antianginal and hypotensive effect of CCBs when used together with thiazide and loop diuretics, ACE inhibitors, β-blockers and nitrates, as well as to enhance their hypotensive effect when used together with α1-blockers, antipsychotics.
Although negative inotropic effects have not generally been observed in amlodipine studies, some CCBs may potentiate the negative inotropic effects of antiarrhythmic drugs known to prolong the QT interval (eg, amiodarone and quinidine).
Amlodipine can also be safely used concomitantly with antibiotics and oral hypoglycemic agents.
A single dose of 100 mg of sildenafil in patients with essential hypertension does not affect the pharmacokinetic parameters of amlodipine.
Repeated use of amlodipine at a dose of 10 mg and atorvastatin at a dose of 80 mg is not accompanied by significant changes in the pharmacokinetics of atorvastatin.
Simvastatin:
simultaneous repeated use of amlodipine at a dose of 10 mg and simvastatin at a dose of 80 mg leads to an increase in simvastatin exposure by 77%. In such cases, the dose of simvastatin should be limited to 20 mg.
Ethanol (beverages containing alcohol):
amlodipine with single and repeated use at a dose of 10 mg does not affect the pharmacokinetics of ethanol.
Antivirals (ritonavir):
increases plasma concentrations of BCC, incl. and amlodipine.
Neuroleptics and isoflurane:
enhancing the hypotensive effect of dihydropyridine derivatives.
Calcium preparations
may reduce the effect of CCBs.
When combined with CCBs and lithium preparations
(no data available for amlodipine), their neurotoxicity may increase (nausea, vomiting, diarrhea, ataxia, tremor, tinnitus).
Studies of the simultaneous use of amlodipine and cyclosporine in healthy volunteers and all groups of patients, with the exception of patients after kidney transplantation, have not been conducted. Various studies of the interaction of amlodipine with cyclosporine in patients after kidney transplantation show that the use of this combination may not lead to any effect or increase the Cmin of cyclosporine to varying degrees, up to 40%. These data should be taken into account and cyclosporine concentrations should be monitored in this group of patients when cyclosporine and amlodipine are co-administered.
Does not affect serum digoxin
and its renal clearance.
Does not significantly affect the action of warfarin
(PV).
Cimetidine:
does not affect the pharmacokinetics of amlodipine.
in vitro studies
of digoxin, phenytoin, warfarin and indomethacin
to plasma proteins Grapefruit juice:
A simultaneous single dose of 240 mg of grapefruit juice and 10 mg of amlodipine orally is not accompanied by a significant change in the pharmacokinetics of amlodipine. However, it is not recommended to use grapefruit juice and amlodipine at the same time, because with genetic polymorphism of the CYP3A4 isoenzyme, it is possible to increase the bioavailability of amlodipine and, as a result, increase the hypotensive effect.
Aluminum or magnesium containing antacids:
their single dose does not have a significant effect on the pharmacokinetics of amlodipine.
CYP3A4 isoenzyme inhibitors:
with simultaneous use of diltiazem at a dose of 180 mg and amlodipine at a dose of 5 mg in elderly patients (69 to 87 years old) with arterial hypertension, an increase in systemic exposure of amlodipine by 57% was observed. Concomitant use of amlodipine and erythromycin in healthy volunteers (18 to 43 years of age) does not lead to significant changes in amlodipine exposure (22% increase in AUC). Although the clinical significance of these effects is unclear, they may be more pronounced in older patients.
Potent inhibitors of the CYP3A4 isoenzyme (for example, ketoconazole, itraconazole) may increase the plasma concentration of amlodipine to a greater extent than diltiazem. Amlodipine and inhibitors of the CYP3A4 isoenzyme should be used with caution.
Clarithromycin:
CYP3A4 isoenzyme inhibitor. Patients taking clarithromycin and amlodipine at the same time have an increased risk of decreased blood pressure. Patients taking this combination are advised to be under close medical supervision.
Inducers of the CYP3A4 isoenzyme:
There is no data on the effect of inducers of the CYP3A4 isoenzyme on the pharmacokinetics of amlodipine. Blood pressure should be carefully monitored while using amlodipine and inducers of the CYP3A4 isoenzyme.
Tacrolimus:
When used simultaneously with amlodipine, there is a risk of increasing the concentration of tacrolimus in the blood plasma. To avoid toxicity of tacrolimus when used concomitantly with amlodipine, the concentration of tacrolimus in the blood plasma of patients should be monitored and the dose of tacrolimus should be adjusted if necessary.