Dear friends, hello!
Today we will talk about hormonal external remedies. There are several dozen of them in the pharmacies’ assortment, and it’s not easy to figure them out on your own.
I hope that this article will be a ray of light for you in the dark realm of topical (i.e. local) steroid drugs, and in just a few minutes you will know the principles of recommendations for drugs in this group, since it is impossible to pay attention to each drug in one article. And it's not necessary.
But I will dwell on some of them in a little more detail.
In this article we will look at:
- How do topical steroids work?
- What determines their therapeutic effect?
- How are they divided?
- What adverse reactions occur when using them?
- Which drugs in this group are safer than others?
- When to recommend which dosage form?
- What should you pay attention to when choosing such a product for a specific buyer?
- How much topical product should I squeeze onto my finger? You will learn the secret of dosing these drugs.
You are ready?
Then let's go!
A little history
The first topical corticosteroid appeared in 1952. It was hydrocortisone ointment. It was immediately picked up by dermatologists and began to be used in their practice for treating various dermatitis, psoriasis, eczema and other skin diseases that occur with itching, redness, swelling, thickening and flaking of the skin.
And everything would be fine, but, unfortunately, hydrocortisone ointment did not always cope with its tasks.
The development of a more effective steroid for topical use continued, and after 2-3 years, one of the scientists came up with the idea of attaching a halogen atom to the original hormone molecule. Halogens are 5 elements of Mendeleev’s periodic table, of which we are only interested in two: fluorine and chlorine.
Initially, fluoride was chosen to increase the anti-inflammatory activity of the topical steroid.
Hooray! The resulting drugs, indeed, were many times superior to their predecessors in anti-inflammatory activity. History does not tell their names. I suspect that it was Sinalar, Flucinar, Sinaflan, Fluorocort.
But fluorinated steroids dramatically increased the incidence of local and systemic adverse reactions.
Later, steroid drugs appeared on the pharmaceutical market containing not fluorine in their molecule, but chlorine, which made these drugs safer.
And then synthetic non-halogenated topical steroids were developed, the effectiveness of which is comparable to fluorinated drugs, and the safety is comparable to the founder of this group, hydrocortisone.
In the practice of a modern dermatologist, topical glucocorticosteroids (TGCS) occupy a special place. With their help, it is possible to quickly cope with the symptoms of inflammation in many dermatoses. Most doctors cannot imagine their daily practice without these medications. A “revolution” in dermatology occurred in 1952, when hydrocortisone was first used to treat dermatitis, which became a turning point in the treatment of inflammatory skin diseases.
In the 60s XX century domestic scientists V.A. Rakhmanov, N.S. Smelov, A.A. Studnitsin noted the positive effect of hydrocortisone ointment in the treatment of eczema, neurodermatitis, and dermatitis. Almost 50 years have passed since then, and topical steroids have maintained their leading position in the treatment of the most common dermatoses all these years. This is due to the fact that TGCS have the following pharmacodynamic effects [1, 2]:
- anti-inflammatory;
- immunosuppressive;
- vasoconstrictor;
- antiproliferative;
- antipruritic.
The mechanism of action of TGCS is associated with hyposensitization of skin cell receptors for histamine and serotonin, blockade of histaminase A2, increased activity of hyaluronidase, which causes a decrease in the permeability of the vascular wall, exudation, itching, as well as normalization of vascular tone, stabilization of cell membranes.
The anti-inflammatory effect of THCS is due to its inhibitory effect on phospholipase A2, which is necessary for the synthesis of arachidonic acid. This inhibition mechanism leads to the suppression of the formation of inflammatory mediators (prostaglandins, leukotrienes, interleukins, complement components, etc.). There is a decrease in the release of lysosomal enzymes. TGCS cause inhibition of the migration of lymphocytes and macrophages, a delay in the proliferation of fibroblasts and collagen synthesis, and a decrease in the number of Langerhans cells and mast cells. All this ensures the clinical effect of TGCS.
In the first years of using topical hydrocortisone preparations, it was noted that they were well tolerated by patients, but their effectiveness in psoriasis and other dermatoses was low [2]. The next step was to add a double bond to the steroid molecule. This is how prednisolone appeared - a drug whose activity is 4 times greater than that of hydrocortisone. In order to increase anti-inflammatory activity, back in the 50s it was proposed to include one or two halogen atoms (fluorine or chlorine) in the corticosteroid molecule. A new stage has begun in the history of the creation of steroids - the synthesis of halogenated drugs.
Betamethasone dipropionate, the second most potent topical steroid after clobetasol propionate, the b-isomer of dexamethasone, has only one fluorine atom, which significantly reduces the risk of side effects, but without reducing anti-inflammatory activity (Fig. 1).
Rice. 1. Betamethasone dipropionate formula.
Currently, the arsenal of TGCS registered in Russia includes more than 40 items. Such an abundance of drugs with similar mechanisms of action creates difficulties when choosing a specific drug for a specific patient with steroid-sensitive dermatosis. It is urgent to create a modern algorithm for topical therapy of steroid-sensitive dermatoses.
Therapy of dermatological diseases with the use of TGCS dictates the need for strict adherence to certain principles for the selection of drugs, their dosage forms and combinations, and their competent use.
The choice of THC should be determined by its pharmacological properties, the form and stage of the pathological process, the presence of complications, general condition, age of the patient and a number of other factors.
The variety of external preparations and their dosage forms is designed for the experience and qualifications of a dermatologist, his ability to rationally use all the possibilities of topical therapy with the correct diagnosis and stage, severity of the inflammatory process.
In case of secondary infection of lesions, combination drugs with antimicrobials, and in some cases with antifungal agents, should be used; in case of hyperkeratotic processes, with keratolytic ones. Less potent THCS is recommended for use on areas with thin skin (face, folds), as well as in children and elderly patients. During pregnancy, it is not recommended to use THCS for a long time and on large areas of the skin (category C).
It is necessary to take into account environmental factors (temperature, humidity) that promote rapid absorption of the drug. The rate and completeness of absorption of THCS is influenced by lipophilicity, which determines systemic bioavailability, features of drug metabolism, strength and concentration of the active substance, features of the drug base of the drug, the additional presence of penetrants, as well as technological features of drug manufacturing [1, 2].
It is known about the increased permeability of the skin of newborn children due to the thin stratum corneum and loose arrangement of keratinizing cells, abundant blood supply and a small subcutaneous fat layer. A significantly higher ratio of body surface area to body weight, changes in the density of glucocorticosteroid receptors and their ability to mediate a hormonal signal, underdevelopment of metabolizing enzyme systems up to the 2-4th week of life.
In old age, the permeability of the skin is also increased due to its thinning, a decrease in the number of blood vessels, and the permeability of the vascular wall. There are disturbances in microcirculation in the skin (stasis, microthrombosis, emptying of capillaries), and changes in the metabolism and excretion of drugs occur [2].
During the penetration of THCS through the skin, a concentration gradient arises between the base of the drug and the stratum corneum of the epidermis, then the distribution coefficient and diffusion coefficient are significant. The speed and completeness of absorption of TGCS through the skin is influenced by the humidity and temperature of the skin, previous skin lesions, and the anatomical localization of the process. Thus, an increase in skin humidity and temperature leads to an increase in transdermal absorption of the drug, which is achieved by an occlusive dressing or hydrophobic ointment. The fastest and most complete absorption of the drug occurs from the surface of the face and skin folds. Systemic absorption of the drug from the face reaches 7%, armpits - 4% [3].
Thus, in order to increase the effectiveness of therapy for patients with steroid-sensitive dermatoses, there is a need to develop an optimized differentiated approach to the prescription of external treatment, taking into account the stage of development of the inflammatory process, the severity of manifestations and the severity of individual clinical symptoms in typical localizations of the diseases.
Bacterial and fungal agents play a significant role in the pathogenesis of allergic dermatoses. The combined composition of THCS drugs makes it possible to influence the underlying disease and secondary flora [4]. Such combination drugs, effective in the treatment of dermatoses complicated by secondary bacterial and/or mycotic infection, include the drug Akriderm GK
[5, 6]. This combination therapy is optimal due to the combination of betamethasone dipropionate 0.05%, gentamicin sulfate 0.1%, clotrimazole 1%.
Cream Akriderm GK
has a hydrophilic emulsion base with an emollient moisturizing effect, contains nipagin (methyl ester of parahydroxybenzoic acid) with an antimicrobial and antiseptic effect and propylene glycol, which potentiates the effect of THCS and enhances the antimicrobial effect of nipagin.
Gentamicin is a broad-spectrum antibiotic from the aminoglycoside group. The antibacterial effect of gentamicin sulfate is manifested against a wide range of pathogens: Staphylococcus
spp.
, Pseudomonas
spp.
, Proteus
spp.
, Esherichia coli , Klebsiella
spp
., Enterococcus faecalis , Shigella
spp
.
and etc.
Clotrimazole is a universal antimycotic agent with activity against dermatophytes and fungi of the genus Candida.
, pathogens of lichen versicolor and erythrasma. The mechanism of action of clotrimazole is to suppress the synthesis of ergosterol (the main part of the cell wall of the fungus), as well as to influence mitochondrial peroxidase enzymes with a subsequent increase in the concentration of hydrogen peroxide, lysis of fungal cells (fungicidal effect) [7, 8].
Pronounced clinical effect of the drug Akriderm GK
due to the high therapeutic activity of each component, a successful combination of active principles in its composition, creating a symbiosis effect. The therapeutic effect has been proven for a number of diseases. The drug is applied to clean skin (except the face) in a thin layer, 2 times a day, without massaging the skin or rubbing the drug. If necessary (for hyperkeratotic processes), the drug can be applied to dry skin after a bath or shower. Long-term anti-relapse treatment is recommended for moderate to severe cases of the process using dermatological emollients from 1 to several times a day.
The results of numerous studies have shown that the combination drug Akriderm GK
has high therapeutic efficacy for chronic dermatoses complicated by bacterial and mycotic infections, the drug allows you to shorten the duration of therapy, increase periods of remission and reduce the number of exacerbations, improve the prognosis of diseases in the absence of side effects [9, 10].
Thus, the most effective as local therapy are combination drugs, which, in addition to TGCS, include antibacterial and antimycotic agents, which makes it possible to simultaneously influence all parts of the pathological process.
Skin areas with a thin skin barrier and reduced barrier function require special protection - eyelids, skin on the back of the ear, face, flexor surfaces of joints. When the inflammatory process is localized on the skin of the face, to speed up the process of restoration of the skin and prevent exacerbations of the disease, soften and moisturize damaged, sensitive, inflamed and dry skin, restore normal water balance and lipid levels of the skin, elasticity, and protect the skin for a long period, products are recommended, having a pronounced moisturizing, softening and regenerating effect. These include a complex of lipids, urea, glycerin and other bioorganic compounds that are part of the natural moisturizing factor (Fig. 2) and provide long-term hydration of the skin, restoration of the hydrolipid film and water balance of damaged skin.
Rice. 2. Natural moisturizing factor components.
The moisturizing effect of urea is due to the absorption of water from the dermis and its retention in epidermal cells, reducing transepidermal moisture loss. Due to its keratolytic effect, urea can act as a conductor for other active substances included in preparations. Also, urea retains its absorbent and antiseptic properties for a long time, preventing the occurrence of infection; no side effects or allergic reactions have been observed when using urea. Researchers have noted positive results from the use of drugs with urea in the treatment of patients with eczema, dermatitis, keratosis, psoriasis, ichthyosis, and atopic dermatitis [11-15].
Modern requirements for topical steroids are as follows: they must be highly effective, confirmed in studies that meet the standards of evidence-based medicine (multicenter randomized clinical trials that meet GCP standards); have a variety of dosage forms for topical use necessary to achieve an effect at different stages of the inflammatory process and on any area of the skin; be safe (low absorption, minimum side effects, possibility of long-term use); be easy to use (application once a day; no odor, no greasy sheen; do not stain clothes).
All of these properties are possessed by methylprednisolone aceponate, a non-halogenated drug from the group of topical steroids, which has an optimal combination of high local activity and minimal systemic and local side effects. This effect of MPA is due to the inclusion of ether side chains in its structure. MPA is a diester (Fig. 3), characterized by high lipophilicity (short chain of fatty acids at positions C17 and C21), which allows it to quickly and easily penetrate through the stratum corneum into the dermis. The absence of fluorine and chlorine at positions C6, C9 or C12 ensures a minimum of local and systemic side effects [16, 17].
Rice. 3. Chemical structure of methylprednisolone aceponate.
The potency of MPA is superior to a number of modern corticosteroid drugs. In contrast, it has virtually no systemic glucocorticoid and mineralocorticoid effects. The process of bioactivation of MPA at the site of inflammation in the skin occurs much faster than in healthy skin. The penetration of MPA and its metabolites into the blood and their half-life in the blood are very insignificant, while the connection with the protein transcortin is quite large, which determines the almost complete absence of systemic side effects in MPA. The higher affinity of MPA metabolites (17-propionate and free methylprednisolone) for skin steroid receptors than that of the parent substance is manifested in long-term, pronounced suppression of the process of inflammation, hyperproliferation and allergic reactions; in addition, researchers noted that one of the products of MPA metabolism is acetic acid acid that has an antiseptic effect [17].
The clinical effectiveness and safety of MPA has been proven in clinical studies involving more than 15,000 adults and children, which allows for an objective assessment of this drug [18-20].
In the treatment of atopic dermatitis (AD) in children using topical steroids, the best results were observed in the latest generation of drugs, which include MPA. When used once daily, MPA is an effective and safe treatment for AD in children [21, 22].
The company AKRIKHIN JSC has created a new drug - Comfoderm M
2: Unique combination of MPA and 2% urea.
The purpose of creating the drug Comfoderm M
2 was to increase the effectiveness of the treatment of steroid-sensitive dermatoses through the combination of TGCS with a moisturizing component - 2% urea. The combined use of MPA and 2% urea is more effective than the separate action of these drugs, which significantly expands the range of application and makes it possible to treat steroid-sensitive dermatoses even in areas with thin sensitive skin: face, neck, folds.
Moderate and severe steroid-sensitive dermatoses, including AD, require long-term control and mild anti-relapse treatment. Topical calcineurin inhibitors are non-steroidal immunomodulators and have a significant effect compared to placebo for both short-term and long-term use. Tacrolimus can be used for the maintenance treatment of moderate to severe AD (2 times a week, for example, on Monday and Thursday), applied to the areas of the skin where lesions most often occur, in patients with frequent exacerbations (more than 4 episodes). per year) in order to prevent new exacerbations and prolong the period of remission. This therapy is indicated only for those patients who have previously responded to treatment with tacrolimus twice a day for no more than 6 weeks (i.e., treatment led to complete or almost complete resolution of the skin process). There are no data on similar use of pimecrolimus. After 12 months of maintenance therapy, it is necessary to assess the dynamics of clinical manifestations and decide on the advisability of continuing the use of tacrolimus [23, 24].
Dermatovenereologists will be interested in a new domestically produced anti-inflammatory drug for topical use - Tacropic
(tacrolimus), which belongs to the group of calcineurin inhibitors.
Inhibition of the phosphatase activity of calcineurin occurs as a result of the connection of Tacropic
with a specific cytoplasmic protein immunophilin (FK506) and the formation of a complex - tacrolimus, FKBP12, calcium, calmodulin and calcineurin. In the multifaceted mechanism of action of tacrolimus, one should also note its inhibitory effect on the release of inflammatory mediators from mast cells, basophils and eosinophils, and the absence of atrophogenic effect.
For long-term control, Tacropic
in a dosage of 0.1% is used in the treatment of blood pressure in adolescents and adults from 16 years of age 2 times a week, in a dosage of 0.03% - in the treatment of blood pressure in children from 2 years of age 2 times a week for up to a year, combination with dermatological emollients is possible means.
For topical treatment of steroid-sensitive dermatoses in children, AKRIKHIN JSC has proposed a new drug - Comfoderm K
— methylprednisolone aceponate 0.1% (MPA) and ceramides, thanks to the hydrophilic base, this remedy can be used even in the acute stage of the inflammatory process.
Ceramides were first isolated from brain tissue. They got their second name - ceramides - from the Latin word cerebrum
(brain). Later it was discovered that ceramides form the basis of the lipid layer between the horny scales of the skin. Ceramides belong to the class of sphingolipids. These are complex lipids consisting of several blocks - the fatty alcohol sphingosine or phytosphingosine (forms a hydrophilic “head”) and one fatty acid (lipophilic “tail”). Among ceramides, long-chain ceramides, which include linoleic acid, stand out. These ceramides “stitch” adjacent lipid layers and bind them into a single structure. With a lack of linolenic acid, the synthesis of ceramides suffers, and accordingly, the lipid layer of the stratum corneum loses its integrity and disintegrates. The consequence of this is dry skin and other associated symptoms (flaking, increased sensitivity, irritation, etc.) [25]. A decrease in the amount of ceramides in the skin is one of the etiological factors of atopic dermatitis (Fig. 4), therefore the inclusion of ceramides in a complex Therapy of atopic dermatitis in children helps alleviate the disease [26].
Rice.
4. Violation of the structure of the lipid composition of the epidermis in atopic dermatitis. When applied externally, Comfoderm K
suppresses inflammatory and allergic skin reactions, increased proliferation, reducing subjective sensations and objective manifestations of inflammation.
The drug is indicated in the topical treatment of steroid-sensitive dermatoses in children from 4 months of age. Comfoderm K
cream is applied in a thin layer to areas of inflammation once a day for no more than 4 weeks for children and up to 12 weeks for adults.
In the chronic stage of the skin inflammatory process in children, 0.1% Comfoderm
(MPA), which also appeared in the new
Comfoderm
. Recommendations for use: 1 time per day for up to 4 weeks together with dermatological emollient agents.
Our many years of experience in treating patients with steroid-sensitive dermatoses shows that the decision to choose between TGCS and a non-steroidal drug, for example, from the group of calcineurin antagonists, in each specific case largely depends on the patient’s condition when he first contacts a dermatologist. We have proposed a modern algorithm for selecting a topical drug for topical therapy of steroid-sensitive dermatoses, taking into account the severity, nature, localization of the pathological process, duration of the disease, previous treatment and its effectiveness, and the patient’s age. When included in the algorithm, we gave preference to modern TGCS with a high safety profile and proven effectiveness (Fig. 5, 6).
Rice. 5. Algorithm for complex topical therapy of steroid-sensitive dermatoses in children.
Rice. 6. Algorithm for complex external therapy of steroid-sensitive dermatoses.
At the dermatological clinic of the Ural Research Institute of Dermatovenereology and Immunopathology, the proposed algorithms were tested in the treatment of patients with various steroid-sensitive dermatoses. Clinical effectiveness allows us to recommend the proposed treatment algorithms for widespread use in the practice of a dermatologist.
It should be emphasized that a differentiated approach to the selection of topical therapy for patients with steroid-sensitive dermatoses, taking into account the severity, nature, localization of the pathological process, duration of the disease, previous treatment and its effectiveness, and the patient’s age, will increase the effectiveness of therapy during the period of exacerbation, avoid undesirable effects, and significantly reduce systemic pharmacological load, achieve stable, long-term remission and, ultimately, significantly improve the quality of life of such patients.
When are topical steroids used?
There are quite a few indications for the use of these drugs. But if we generalize them, then these are all chronic skin diseases, which are based on inflammation or allergies, or increased division of cells in the stratum corneum of the skin.
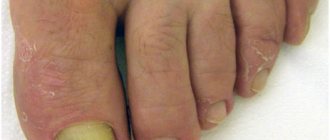
They can manifest themselves as redness, itching, weeping, excessive dryness of the skin, peeling, and the formation of small blisters.
These are all kinds of dermatitis (atopic, seborrheic, contact, solar), psoriasis, lichen planus.
The exception is perioral dermatitis. It manifests itself as pimples and redness around the mouth. In this form of dermatitis, the use of external steroid agents is contraindicated. There has been a connection between the use of topical steroids and the appearance of perioral dermatitis after their withdrawal. The reasons are unclear.
Local corticosteroids are also used for severe insect bites, severe senile itching, and anogenital itching.
There is a nuance regarding anogenital itching. For example, old drugs (Sinaflan, Flucinar, etc.) are contraindicated for this problem. They are not intended for application to mucous membranes, because... high risk of systemic effects.
Possibilities of systemic and topical steroids in the treatment of inflammatory bowel diseases in children
Inflammatory bowel diseases (IBD), which include Crohn's disease (CD) and ulcerative colitis (UC), are the most severe pathology of the gastrointestinal tract (GIT) and are characterized by a chronic, steadily progressive course with the risk of developing intestinal and extraintestinal complications. In approximately one third of patients, the disease first manifests itself before the age of 18 years. In recent years, in developed countries of Europe and North America, there has been a steady increase in the incidence of IBD, the prevalence of which in children varies from 3.4 to 7.1:100?000 [1]. In Russia, an increase in incidence has only emerged in recent years, but this increase has turned out to be very significant. According to our data, the incidence among children in St. Petersburg is approximately 2:100,000 per year, and the prevalence is 6:100,000, that is, it corresponds to the European one. If the incidence of UC in children has remained virtually unchanged over the past 7 years and amounted to 0.4:100?000, then the incidence of CD is steadily growing almost exponentially: for example, in 2002 it was 0.125:100?000, and in 2008 g.?—1.7:100?000 of the child population of St. Petersburg, 4 times higher than the incidence of UC.
The etiology of IBD remains unknown, but breakdown of immunological tolerance to intestinal antigens is thought to be a key factor in the pathogenesis. As a result, immunological control over the process of progressive inflammation in the intestine is lost. Due to the fact that inflammation is the dominant mechanism of development of both UC and CD, exacerbations of both diseases are treated with drugs with anti-inflammatory effects: 5-aminosalicylic acid (5-ASA) or glucocorticosteroids (GCS). According to our data, at least 70% of children with IBD require GCS therapy.
In the physiological state, endogenous glucocorticoids counteract the activation of the innate and adaptive immune responses [1, 2]. Low doses of exogenous steroids may also follow this physiological pathway, but when given in high doses, corticosteroids involve other mechanisms of action. After passive diffusion into the cell, they bind to specific cytoplasmic glucocorticoid receptors, which are present in most cells of the body in the number of 2000 to 30,000 binding sites. This complex then enters the cell nucleus and comes into contact with glucocorticoid response elements (GREs) of DNA in the region of specific genes. As a result, suppression of genes encoding the transcription of inflammatory proteins occurs, in particular, signaling molecules of the MAPK (mitogen-activated protein kinase) cascade. In parallel, the synthesis of IkBa, an inhibitor of the key transcription factor NFkB, is enhanced, thereby suppressing the formation of specific transport RNAs (tRNAs) and shortening their half-life. Since tRNAs are responsible for regulating the synthesis and release of pro-inflammatory cytokines TNF-alpha, IFN-gamma, IL-23, IL-17 and others involved in the implementation of the inflammatory response in IBD, their level decreases when GCS is prescribed. This also reduces the formation of arachidonic acid and its subsequent metabolism to form leukotrienes and prostaglandins [3].
The direct effect of GCS on the transcription of genes that activate inflammation plays a major role in achieving the anti-inflammatory effect, but is not limited to it. Nongenetic mechanisms include inhibition of endothelial NO synthetase activation. NO is one of the important participants in the inflammatory cascade in IBD, influencing the leukocyte-endothelial interaction and promoting vasodilation and microcirculatory disorders. GCS activate phosphorylation processes and facilitate the entry of calcium into cells [4].
Thus, GCS, interacting with various molecules, have a multifaceted effect, which through a number of mechanisms is realized in a powerful anti-inflammatory effect.
Systemic corticosteroids (prednisolone, hydrocortisone, methylprednisolone, etc.) have been used in the treatment of IBD for several decades. Typically, patients with IBD respond well to systemic steroid therapy, and although placebo-controlled studies of the effectiveness and safety of prednisolone in this pathology in children have not been conducted, comparison with enteral artificial nutrition, as the only method of therapy, has shown that the effectiveness of systemic steroids in CD reaches 85%. [5], with UC - 82% [6].
The patient's response to GCS therapy, including hormone resistance, depends on the genetically determined density of glucocorticoid receptors, as well as on the affinity of a particular GCS for them. If the first component is purely individual and associated with mutations in the GCS receptor gene, then the second can be changed by optimal choice of drug. Comparative studies have shown that budesonide is the drug with the highest affinity. Topical activity, that is, activity in the field of release of the active substance, depends on affinity: the higher the affinity, the higher the local effect. Thus, budesonide can be considered one of the most effective topical steroids [7].
It is well known that long courses of systemic corticosteroids are associated with the development of a number of undesirable side effects: moon face, stretch marks, acne, hypertrichosis, increased blood pressure, hyperglycemia, osteoporosis, etc. Hormone dependence is also a serious problem: 31% of children with CD and 45% with UC were hormone dependent a year after diagnosis, which required the administration of cytostatic therapy [8, 9]. This is consistent with data obtained in adult patients [10]. In this regard, in the treatment of local inflammatory processes in recent years, preference has been given to hormonal drugs with the lowest systemic bioavailability and predominant local action, such as budesonide, beclomethasone, fluticasone and hydrocortisone. This approach has become generally accepted in the treatment of diseases such as bronchial asthma and allergic rhinitis, but has not yet found widespread use in gastroenterology. Only in recent years, the budesonide drug Budenofalk®, containing 3 mg of the active substance in each capsule, has been used in the treatment of IBD. Budenofalk® capsules dissolve in the stomach, but each contains about 350 granules with a diameter of 1 mm, coated with an acid-resistant coating containing Eudragit S. The coating is destroyed at a pH above 6.4, which corresponds to the level of the terminal ileum. Therefore, the maximum effect of the drug is achieved in the ileocecal region. Since budesonide in the drug is presented in an active form, it begins to act immediately after release. Due to its chemical structure, namely C-16,17 alpha acetonide, budesonide is highly lipophilic and quickly penetrates cell membranes.
Classic corticosteroids (hydrocortisone, prednisolone, methylprednisolone, dexamethasone, etc.) have high systemic bioavailability, while this indicator for budesonide is very low [11]. The low systemic bioavailability of budesonide is a consequence of gastrointestinal uptake regulated by P-glycoprotein, the product of the MDR1 (multidrug resistance) gene, and biotransformation in the liver by cytochrome p450 3A (CYP 3A). When budesonide first enters the liver, its metabolites are formed: 6-beta-hydroxybudesonide and 16-alpha-hydroxyprednisolone, the glucocorticoid activity of which is only 1–10% of the activity of budesonide absorbed into the blood. The systemic bioavailability of budesonide in children (9 ± 5%) is close to that in adults (11 ± 7%) [12], respectively, systemic elimination, calculated from the half-life and clearance of the drug, does not differ in adults and children [13].
Conversion of the drug in the liver to 6-beta-hydroxybudesonide occurs 1.5 times faster in children than in adults, reflecting higher activity of CYP3A enzymes in the liver [13]. Due to these pharmacodynamic features, budesonide (Budenofalk®) does not require calculation of the dose per kilogram of weight or body surface area; the same dose is used in both adults and children, the starting dose of the drug is 9 mg per day. Since the side effects of GCS are due to their systemic action, the pharmacokinetics of budesonide, consisting of a combination of high affinity for local GCS receptors and low systemic bioavailability, create unique properties for the drug. Budesonide has a pronounced local anti-inflammatory effect with a minimal number of adverse reactions, which is confirmed by clinical studies, but there is little experience with the use of this drug in pediatric practice.
The purpose of our work was to evaluate the effectiveness of budesonide (Budenofalk®) in children with IBD in comparison with therapy with mesalazine and prednisolone.
Material and methods: we observed 37 patients (24 with UC and 13 with CD) aged from 8 to 17 years (average age 14.3 years). The diagnosis of IBD was made for the first time in all patients. By the time treatment began, all children were in the stage of exacerbation of the disease. UC in all patients had moderate activity and was total in nature, CD was localized in the ileocecal region, and in 6 patients it affected other parts of the colon. Extraintestinal manifestations in the form of primary sclerosing cholangitis were diagnosed in 2 patients with UC, arthritis - in 1 patient with UC and 1 patient with CD. Patients were randomized into groups: 12 patients received mesalazine (Salofalk) at a dose of 50 mg/kg/s , 14 - prednisolone at a dose of 1 mg/kg/s for 2 weeks, followed by a gradual reduction to a maintenance dose of 5 mg/s, 11 children (4 with CD and 7 with UC) received Budenofalk® at a dose of 9 mg/s for 2 months followed by reduction to 6 mg/s.
Patients were monitored for a year with assessments at 1, 3, 6 and 12 months from the start of treatment. At the same time, in dynamics, a score was made for the severity of intestinal syndrome (CS), general clinical activity (GCA), endoscopic activity (E), laboratory activity (L), protein-calorie deficiency (BCN), dynamics of extraintestinal manifestations (EP) if present, all data were summarized as a total score (S).
Results and its discussion
During treatment with Salofalk, a decrease in the manifestations of CS and E was achieved after 3 months, while the difference compared with the initial indicator was on average 3.0 ± 0.4 points for both CS and E. Further treatment was accompanied by a slower rate of decline activity, endoscopic remission was achieved in 60% of patients. The administration of prednisolone was accompanied by a faster rate of decline in activity; after 1 month, the CS decreased by 7.1 ± 1.2 points, E by 8.2 ± 0.8 points, L by 5.1 ± 0.7 points, however after reducing the dose during maintenance therapy, already after 3 months there was a gradual increase in the activity of CS and E by an average of 2.3 ± 0.3 points. During treatment with Budenofalk®, the decrease in activity occurred at a slower pace than during prednisolone; after 3 months, the CS decreased by 3.6 ± 0.7 points, E - by 6.2 ± 0.8 points, L - by 5.4 ± 0.7 points.
In the subsequent months, no deterioration of the condition was noted; on the contrary, a further decrease in activity was observed; after 6 months from the start of treatment, remission was achieved in 7 out of 11 patients (64%). Remission within 6 months of treatment was achieved in 50% of patients receiving Salofalk, 57% - Prednisolone and 67% - Budenofalk® (), relapse of the disease within a year was observed in 8% of children receiving Salofalk, 64% - maintenance therapy with Prednisolone and 18% - Budenofalkom®. Thus, the effectiveness of the topical steroid after 6 months was the highest in comparison with 5-SK and systemic corticosteroids, and its preventive effect in maintaining remission after 1 year was close to that of Salofalk. It should be noted, however, that too early a reduction in the dose of Budenofalk® from 9 mg to 6 mg (after 1–2 months), given the gradual onset of the effect while taking it, is associated with the risk of exacerbation, therefore it is advisable to prescribe the full dose for 6 months, until complete remission occurs, and only after that gradually reduce it. In this case, not only a therapeutic, but also an anti-relapse effect of the drug is achieved. Intensified therapy in the form of switching to systemic corticosteroids or increasing their dose was required in 42% of patients receiving Salofalk, 7% - Prednisolone and 18% - Budenofalk®; cytostatics were prescribed to 43% of patients on the background of Prednisolone and only 18% - on the background of Budenofalk® .
The most significant differences were found in the number and severity of adverse reactions to therapy. In 27% of children receiving Budenofalk®, only a slight moon-shaped face was noted, while with Prednisolone, adverse reactions were observed in all patients (), and adverse reactions were also noted in 11% of patients receiving Salofalk. Thus, our study confirmed the fairly high effectiveness of Budenofalk® in relation to both patients with CD and UC with moderate activity. The drug showed comparable efficacy to systemic steroids with significantly greater safety, which is especially important in childhood. Our data are consistent with the results of two randomized clinical trials comparing the effectiveness and safety of systemic (Prednisolone) and topical (budesonide) GCS, which were conducted in children. In the work of A.?Levin et al. [14] 33 patients (mean age 14.3 years) with mild or moderate active CD were randomized into two groups, one of which received Budenofalk® at a dose of 9 mg/s for 12 weeks, and the other received Prednisolone at a dose of 40 mg/s. The groups did not differ in age, location, activity and duration of the disease. Remission, calculated by the Pediatric CD Activity Index (PCDAI < 10) at week 12 of treatment, was achieved in 9 of 19 (47%) children receiving Budenofalk®, and 7 of 14 (50%) receiving Prednisolone. However, side effects were observed in 71% of children receiving Prednisolone, and only 32% receiving Budenofalk®. The severity of cosmetic side effects was significantly lower when Budenofalk® was prescribed.
J.?C.?Escher et al. [15] conducted a controlled, multicenter, randomized, double-blind study. Through the joint efforts of the ESPGHAN IBD Working Group, 36 centers from 8 European countries took part. The study included 48 patients with newly diagnosed active CD with ileocecal localization, whose average age was 13 years. Patients were randomized into two groups, one of which received budesonide at a dose of 9 mg/s for 12 weeks, the second received Prednisolone at a dose of 1 mg/kg/s in the first 4 weeks, followed by a decrease over 4 weeks to 2.5 mg. /With. The first efficacy assessment was carried out after 8 weeks of treatment to achieve remission (Best index < 150). After 2 weeks of treatment, 50% of children in each group achieved remission. By the 8th week, remission in the group of children receiving Prednisolone was observed in 71%, budesonide in 55%, after 12 weeks remission remained in 55% of patients receiving budesonide, and decreased to 60% in the group of children receiving prednisolone. Thus, as in the study by A.?Levin, no significant differences were found in the effectiveness of systemic and topical steroids in the treatment of active CD in children.
Side effects of budesonide, according to a multicenter study by J.?C.?Escher [15], were observed in children much less frequently than when prescribing Prednisolone, as in our study, only a slight moon-shaped face, occasionally hirsutism and mood changes were noted. Suppression of the function of the adrenal cortex, according to morning plasma cortisol levels, was practically not observed when budesonide was prescribed, in contrast to Prednisolone. A retrospective assessment of the growth of prepubertal children treated with budesonide showed that their growth rate corresponded to the lower age limit of normal (2 cm/year) [16].
A meta-analysis of studies conducted in adults showed that budesonide is more effective than mesalazine, but equal or slightly inferior to the effectiveness of systemic corticosteroids in the treatment of CD with ileocecal localization of moderate activity [17]. However, all studies conducted in adults demonstrated significantly fewer side effects from budesonide compared to systemic corticosteroids. Based on these data, the European Association for the Study of CD and UC (ECCO) recommends budesonide as the drug of choice for ileocecal localization of CD with weak or moderate activity [5].
Maintenance therapy with budesonide in remission has not been studied prospectively in children. Systemic corticosteroids in low doses were ineffective in maintaining remission, and since their long-term use is associated with impaired growth and bone mineralization in children, the recommended course of systemic corticosteroids for exacerbation of IBD should not exceed 3 months. A meta-analysis of 4 randomized placebo-controlled trials in adults with CD showed that maintenance therapy with budesonide 6 mg/s is not effective in maintaining remission [18] and is not currently recommended in children. Our results allow us to recommend long-term courses of Budenofalk® (at least 6 months at full dose with subsequent reduction), in which not only the anti-inflammatory, but also the anti-relapse effect of the drug is realized. Taking into account the high safety profile noted by both us and foreign researchers, long-term administration of Budenofalk® (up to one year) can be used in children with IBD without the risk of developing undesirable side reactions.
conclusions
- The use of topical steroids (Budenofalk®) is effective for moderate activity of Crohn's disease and nonspecific ulcerative colitis in children.
- Budenofalk® more effectively reduces intestinal, but not systemic, manifestations of IBD.
- Adverse reactions during treatment with Budenofalk® are mild and are limited to a slight moon-shaped change in the face.
- Budenofalk® should be prescribed at a starting dose of 9 mg/day for 6 months and the dose should be reduced only after complete remission occurs.
- Budenofalk® in a maintenance dose may have an anti-relapse effect.
Literature
- Mamula P., Marcowitz J.?E., Baldassano R.?N. Pediatric Inflammatory Bowel Disease. Springer, 2007, 662 p.
- Marcowitz J., Grancher K., Kohn N. et al. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn disease // Gastroenterology, 2000, v. 119, s. 4, p. 895–902.
- Rhen T., Cidlowski J.?A. Antiinflammatory action of glucocorticoids - new mechanisms for old drugs // N.?Engl. J.?Med., 2005, v. 353 (s. 16), 1711–1723.
- Barnes P.?J., Adcock I.?M. How do corticosteroids work in asthma? //Ann.Intern. Med., 2003, v. 139 (s.5 pt1), p. 359–370.
- Travis S.?P., Stange E.?F., Lemann M. et al. European evidence based consensus on the diagnosis and management of Crohn disease: current management // Gut, 2006, v. 55, s. 1, p. 16–35.
- Travis S.?P., Stange E.?F., Lemann M. et al. European evidence based consensus on the management of ulcerative colitis: current management // J. of Crohn's and Colitis, 2008, v. 2, p. 24–62.
- Mollmann H.?W., May B. et al. Glucocorticoid therapy in chronic inflammatory bowel disease - from basic principles to rational therapy.Kluwer academic publishers, Dordrecht, Boston, London, 1996, p.42–60
- Marcowitz J., Hyams J., Mack D. et al. Corticosteroid therapy in the age of infliximab: acute and 1-year outcomes in newly diagnosed children with Crohn disease.?— Clin.Gasteroenterol. Hepatol., 2006, v.4 (s.9), p.1124–1129.
- Hyams J., Marcowitz J., Lerer T. et al. The natural history of corticosteroid therapy for ulcerative colitis in children // Clin Gastroenterol. Hepatol., 2006, v. 4, s. 9, p. 1118–1123.
- Faubion W.?A., Loflus E.?V., Harmsen W.?S. et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study // Gastroenterology, 2001, v. 121 (s. 2), p. 255–260.
- Brattsand R. Steroid development: a case of enhanced selectivity for the bowel wall // Res. Clin. Forums, 1993, v. 15, p. 17–31.
- Lundin P.?D., Edsbacker S., Bergstrand M. et al. Pharmacokinetics of budesonide controlled ileal release capsules in children and adults with active Crohn disease // Aliment. Pharmacol. Ther., 2003, v. 17 (1), p. 85–92.
- Dilger K., Alberer M., Busch A. et al. Pharmacokinetics and pharmacodynamic action of budesonide in children with Crohn disease // Aliment. Pharmacol. Ther., 2006, v. 23(3), p. 387–396.
- Levine A., Weizman Z., Broide E. et al. A comparison of budesonide and prednisone for the treatment of active pediatric Crohn disease // J.?Pediatric. Gastroenterol. Nutr., 2003, v. 36(2), p. 248–252.
- Escher J.?C. Budesonide versus prednisolone for treatment of active Crohn disease in children: a randomized, double-blind<controlled, multicentre trial//Eur.J.Gastroenterol. Hepatol., 2004, v.16 (1), p. 47–54
- Kundhal P., Zachos M., Holmes J.?L., Griffiths A.?M. Controlled ileal release budesonide in pediatric Crohn disease: efficacy and effect on growth // J.?Pediatr. Gastroenterol. Nutr., 2001, v. 33(1), p.75–80.
- Otley A., Steinhart A.?H. Budesonide for induction of remission in Crohn disease // Cochrane Database Syst. Rev., 2005 (4): CD000296.
- Simms L., Stenhart A.?H. Budesonide for maintenance of remission in Crohn disease // Cochrane Database Syst. Rev., 2001(1):CD002913.
E. A. Kornienko *, Doctor of Medical Sciences, Professor E. A. Lomakina * N. K. Zaletova * S. A. Fadina **
* St. Petersburg State Pediatric Medical Academy of Roszdrav , ** Children's City Clinical Hospital No. 5 named after. N. F. Filatova , St. Petersburg
Contact information for authors for correspondence
How do topical steroids work?
To put it simply, topical steroids penetrate skin cells and bind to the steroid receptors there. After this, a cascade of reactions is launched, as a result of which the release of biologically active substances that cause an inflammatory reaction is reduced. In particular, the release of histamine, the culprit of inflammatory and allergic reactions, decreases, blood vessels constrict, itching, swelling, and redness decrease.
But that is not all. Topical steroids inhibit skin cell division, which in some diseases gets out of control and needs to be curbed. For example, with psoriasis.
But at the same time they suppress local immunity. The protective function of the skin is reduced, and therefore in the list of adverse reactions we can read:
- Steroid acne,
- Activation of a viral, bacterial or fungal infection,
- Delayed regeneration of wounds, trophic ulcers.
This is not a complete list of adverse reactions that external steroids can cause.
In addition to reducing local immunity, hormones suppress fibroblast function. Fibroblasts are the most important skin cells, because... they produce everything she needs for complete happiness. Well, for us too, of course.
These are hyaluronic acid, collagen, elastin, which are responsible for hydration, firmness and elasticity of the skin.
If fibroblast function is suppressed, the skin simply atrophies. Externally, atrophied skin looks shiny, wrinkled, and small intradermal and subcutaneous vessels begin to appear through it, i.e. telangiectasia appears.
This also explains the possible appearance of stretch marks (stretch marks).
How do anabolic steroids affect the brain?
Anabolic steroids work differently than other drugs; they have no short-term effects on the brain. The most important difference is that steroids do not directly activate the reward system, nor do they cause a rapid increase in the brain chemical dopamine, which most drugs increase.
Misuse of anabolic steroids can lead to negative mental effects such as:
paranoid (extreme, unreasonable) jealousy
extreme irritability and aggression (“mad rage”)
delusions - false beliefs or ideas; impaired judgment
thinking disorder
mania
Other adverse reactions
Sometimes external steroids cause tachyphylaxis. This term means that the effectiveness of the drug decreases with repeated use.
After prolonged use of corticosteroids, withdrawal syndrome is possible, when all the symptoms flare up with renewed vigor. It turns out to be a vicious circle.
In some cases, drugs in this group cause allergic dermatitis.
Serious consequences include suppression of adrenal function and the development of Itsenko-Cushing syndrome (moon-shaped face, obesity, swelling of the extremities, etc.), adrenal insufficiency, and the development of steroid gastric ulcers.
In children, long-term use of drugs in this group leads to impaired secretion of growth hormone and growth retardation.
So, when, when a buyer complains about skin itching and flaking, your hand immediately reaches for Sinaflan, remember this article.
How are topical corticosteroids divided?
Classification by activity:
The principle of prescribing a topical steroid based on activity:
- Weak steroids are prescribed for mild inflammatory manifestations. Previously, before the advent of modern topical steroids, they were used for localized inflammation on the face, neck, and in the area of folds, because there is the thinnest epidermis, a high density of steroid receptors and many blood vessels. Therefore, the risk of adverse reactions is higher.
- Medications of moderate activity are indicated in the absence of effect from class 1 drugs and in cases of more severe inflammation.
- Strong topical steroids are used for severe inflammation: skin redness, swelling, areas of induration (plaques), severe itching, and thickening of the skin (hyperkeratosis).
- Very strong steroids are prescribed, as a rule, when the drugs of the first three groups are ineffective.
Classification of local corticosteroids by composition
Based on their composition, topical steroids are divided into halogenated and non-halogenated.
Halogenated - those whose molecule contains one or more halogen atoms (fluorine or chlorine).
Halogenated ones give more unwanted reactions, because the halogen in the active substance molecule slows down the metabolism of the steroid, and it exists longer in its active form. In addition, they bind poorly to transport proteins and are eliminated from the body more slowly.
Older drugs are especially guilty of this: Flucinar, Sinaflan, Fluorocort.
Halogenated ones, in turn, are divided into fluorine-containing and chlorine-containing.
Fluorine -containing ones are Lorinden, Ftorokort, Triakort, Sinaflan, Flucinar, Akriderm, Celestoderm-V, Beloderm, Dermovate.
Fluoride-containing drugs are more likely than others to cause skin atrophy with long-term use.
containing ones are Elokom, Afloderm. If we compare them with fluoride-containing ones, the therapeutic effect of their use occurs faster and they are safer.
Non-halogenated ones are Advantan and Lokoid. They are characterized by a low frequency of adverse reactions and minimal systemic effect.
Publications
The experience of using topical steroids in dermatological practice for the treatment of common forms of dermatoses, such as atopic dermatitis (AD), eczema, psoriasis, goes back more than 50 years. However, the problem of choosing the optimal means of external therapy is still relevant today.
Modern therapy of allergic dermatoses, along with elimination and dietary measures, as well as medications whose action is aimed at various stages of pathogenesis, includes the use of external agents. Local therapy is tailored individually, taking into account the period of the disease, its clinical and morphological form, the presence of concomitant skin changes and complications. The correct choice in the treatment of complicated forms of allergic dermatoses is especially important due to the fact that various factors (bacterial, fungal, etc.) are involved in the pathogenesis of the disease. The consensus of dermatologists and pediatricians provides for early external therapy using topical corticosteroids for severe exacerbation of the skin process, long-term disease control, safe effective therapy that simultaneously provides early relief of symptoms and long-term control.
The mechanism of the anti-inflammatory effect of glucocorticosteroids (GCS) is explained by a wide range of immunoregulatory effects on cells responsible for the development and maintenance of allergic inflammation of the skin (Langerhans cells, lymphocytes, eosinophils, macrophages, mast cells and others) and a vasoconstrictor effect on skin vessels, reducing swelling. The use of topical corticosteroids suppresses 3 main components of the development of allergic inflammation: the release of mediators, the migration of cells into the affected area and their proliferation.
Anti-inflammatory activity and severity of side effects from the use of GCS depend on the structure of their molecule and the dosage form of the drug. One of the first GCS for external use, hydrocortisone is a weak external anti-inflammatory drug, since it penetrates slowly through the epidermis and does not bind strongly enough to the GCS receptors of cells, which ensure transport of the drug into the cell nucleus and its further pharmacological activity. In this regard, more active synthetic derivatives were created: esterified (the steroid molecule is modified by introducing fatty acid esters) and halogenated (halogens are included - fluorine or chlorine).
The introduction of fluorine into the hydrocortisone molecule made it possible to significantly increase the anti-inflammatory activity of the drugs. However, the increase in therapeutic activity led to a parallel increase in the severity of local side effects. Undesirable effects are due to the high ability of fluorinated corticosteroids to bind to the corresponding receptors not only of immunocompetent cells involved in inflammation, but also of other skin cells, causing atrophic processes in the skin with long-term use. The use of fluoride-containing corticosteroids in large volumes (on large lesions) can lead to systemic effects due to suppression of the production of endogenous glucocorticosteroids. The modern classification of corticosteroids for external use divides them into 7 main groups.
| Power of action | Group | A drug | Release form | Commercial name | % active substance | |
| Very strong | 1 | Clobetasol propionate | cream, ointment, solution | Psoriderm | 0,05 | |
| Betamethasone dipropionate | cream, ointment | Diprolene | 0,05 | |||
| Diflorazone diacetate | ointment | Psorcon | 0,05 | |||
| Strong | 2 | Mometasone furoate | ointment | Elocom | 0,1 | |
| Betamethasone dipropionate | ointment | Diprosone | 0,05 | |||
| Amcinonide | ointment | Cyclocort | 0,1 | |||
| Diflorazone diacetate | ointment | Florone, Maxiflor | 0,05 | |||
| Galcinonide | cream | Halog | 0,1 | |||
| Fluocinonide | cream, gel, ointment | Lidex | 0,05 | |||
| Deoxymethasone | cream, gel, ointment | Topicort | 0,25 | |||
| 3 | Betamethasone dipropionate | cream | Diprosone | 0,05 | ||
| Amcinonide | cream, lotion | Cyclocort | 0,1 | |||
| Triamcinolone acetonide | Cream, ointment | Neoderm, Fluorocort | 0,1 | |||
| Diflorazone diacetate | cream | Florone | 0,05 | |||
| Galcinonide | ointment | Halog | 0,1 | |||
| Betamethasone valerate | ointment | Celestoderm, Valison | 0,1 | |||
| Moderately strong | 4 | Flurandrenolide | ointment | Cordran | 0,05 | |
| Mometasone furoate | cream | Elocom | 0,1 | |||
| Triamcinolone acetonide | cream | Kenalog | 0,1 | |||
| Fluocinolone acetonide | ointment | Flucinar, Sinaflan | 0,025 | |||
| Hydrocortisone valerate | ointment | Westcourt | 0,2 | |||
| 5 | Flurandrenolide | cream | Cordran | 0,05 | ||
| Betamethasone dipropionate | lotion | Diprosone | 0,05 | |||
| Mometasone furoate | lotion | Elocom | 0,1 | |||
| Betamethasone valerate | cream | Celestoderm, Valison | 0,1 | |||
| Hydrocortisone butyrate | cream | Locoid | 0,1 | |||
| Triamcinolone acetonide | lotion | Kenalog | 0,1 | |||
| Fluocinolone acetonide | cream | Synalar, Flucinar | 0,025 | |||
| Hydrocortisone valerate | cream | Westcourt | 0,2 | |||
| Weak | 6 | Betamethasone valerate | lotion | Valison | 0,05 | |
| Triamcinolone acetonide | cream | Aristocort | 0,1 | |||
| Desonide | cream | DesOwen, Tridesilone | 0,05 | |||
| Fluocinolone acetonide | cream, solution | Synalar | 0,01 | |||
| Aclometasone dipropionate | cream, ointment | Aclovate | 0,05 | |||
| 7 | Topical steroids based on hydrocortisone, dexamethasone, flumethalone, prednisolone and methylprednisolone | |||||
In the treatment of complicated forms of AD and microbial (mycotic, paratraumatic, nummular, impetiginous) eczema, the most effective are combined corticosteroids for external use. The following groups of drugs are distinguished:
- GCS (hydrocortisone, prednisolone, depersolone) weak and moderate-strong in combination with antibiotics (oxytetracycline, chloramphenicol, etc.); for example - hyoxysone (contains hydrocortisone acetate and oxytetracycline hydrochloride), oxycort (contains hydrocortisone and oxytetracycline);
- strong corticosteroids in combination with AB - sinalar N (fluocinolone and neomycin), celestoderm B (betamethasone valerate, garamicin), diprogent (betamethasone dipropionate and gentamicin sulfate), vipsogal (betamethasone dipropionate, fluocinolone acetonide, gentamicin sulfate, salicylic acid, panthenol );
- GCS weak and moderate-strong in combination with antiseptics (chlorhexidine, sulfur, iodoxyquinoline), for example - sibicort (hydrocortisone and chlorhexidine), aurobin (prednisolone capronate, triclosan, lidocaine), cortomycetin (hydrocortisone acetate and chloramphenicol), sulfodecortem (hydrocortisone acetate and precipitated sulfur), dermosolone (iodohydroxyquinoline and prednisolone). Combinations of strong corticosteroids for topical use are known: lorindene C (iodohydroxyquinoline and flumethasone), sinalar K (fluocinolone and clioquinol), flucinar (fluocinolone and clioquinol), sikorten plus (halomethasone and triclosan);
— GCS weak and moderate-strong in combination with antifungal substances (miconazole, natamycin), for example, pimafucort (contains hydrocortisone, natamycin and neomycin), mycozolon (miconazole and deperzolon (mazipredone);
- Strong corticosteroids in combination with antifungal substances - travocort (isoconazole nitrate and diflucortolone valerate).
Considering the fact that both microbial and fungal agents play a role in the pathogenesis of various forms of microbial eczema, complicated forms of mycotic infection, and pustular psoriasis, the most effective corticosteroids for external use will be:
- drugs containing both antibacterial and antifungal agents - Neoderm (gramicidin, neomycin sulfate, nystatin, triamcinolone acetonide).
Indications for the use of the drug are dermatoses sensitive to treatment with corticosteroids, as well as secondarily infected with bacteria or fungi, including atopic dermatitis, seborrheic dermatitis, Malassezia infections, athlete's foot inguinal, athlete's foot, mixed forms of intertrigo pedis, psoriasis, allergic contact dermatitis, microbial eczema. Neoderm cream can be used on wet surfaces and diaper rash. Neoderm cream combines the anti-inflammatory, antipruritic, antiallergic and antiexudative effect of triamcinolone acetonide with the antifungal activity of nystatin and the broad antibacterial effect of neomycin sulfate and gramicidin. Nystatin has an antifungal and antibacterial effect (it is an antifungal antibiotic belonging to the group of macrolide polyenes). It has a predominantly fungistatic effect due to binding to ergosterol and damaging the barrier function of the fungal membrane. Active against Candida albicans, as well as fungi of the genus Aspergillus. Neomycin sulfate, a broad-spectrum antibiotic from the aminoglycoside group, acts bactericidal and provides highly effective local treatment of primary and secondary bacterial skin infections. Active against gram-negative microorganisms: Pseudomonas aeruginosa, Aerobacter aerogenes, Escherichia coli, Proteus vulgaris, Klebsiella pneumoniae; gram-positive microorganisms: Streptococcus spp. (susceptible strains of group A alpha and beta hemolytic streptococcus), Staphylococcus aureus (coagulase-positive, coagulase-negative and some penicillinase-producing strains). Side effects may include a contact allergic reaction to neomycin. The risk of developing side effects increases when using external corticosteroids containing antibiotics for more than 7-10 days, which requires the construction of rational medical tactics regarding the external treatment of patients with dermatoses, the timely use of “pure” corticosteroids or other means of external therapy. Gramicidin is an antibiotic that has both bacteriostatic and bactericidal effects and is active against Streptococcus spp., Staphylococcus spp., and anaerobic bacteria, which is especially important in the treatment of lesions of the folds. There is also literature data on the effectiveness of Neoderm in external therapy of patients with pustular psoriasis. This is explained by the characteristics of the microbial flora of the skin and local immune changes, as well as the advisability of influencing not only bacteria, but also fungal microorganisms.
In order to determine the effectiveness of the drug " Neoderm " for external treatment of complicated forms of eczema in adults, a group of 29 people (18 men, 11 women) suffering from various forms of eczema was formed: 13 - microbial nummular eczema, 10 - mycotic eczema of the feet, 3 - paratraumatic eczema, 2 - impetiginous eczema of the legs, 1 - varicose eczema of the legs. In 24 patients, the eczematous process was in the acute stage and was accompanied by pronounced vesiculation and weeping; in the rest, eczema was in the subacute stage, accompanied by erythema and infiltration in the foci. In addition to external therapy, all patients received hyposensitizing and antihistamine drugs orally.
Systemic corticosteroids were not prescribed. The drug " Neoderm " was used 2 times a day for 7-10 days. During therapy, there was a pronounced regression of the clinical symptoms of the disease by the 3-4th day of treatment (wetting regressed on the 3rd day of therapy in 21 out of 24 patients, symptoms of acute inflammation decreased significantly in all patients on the 7th day of treatment, infiltration on the 10th day of treatment). On the 1st day of therapy, only 5 patients retained it. Subsequently, patients received symptomatic anti-inflammatory external therapy. There were no side effects when using the drug "Neoderm". Thus, Neoderm is highly effective for combined skin lesions caused by a mixed infection and accompanied by a pronounced inflammatory reaction. This allows us to recommend it for widespread use in dermatological practice as an anti-inflammatory, antibacterial and antimycotic agent as the first stage of external therapy for dermatoses.
Also in modern literature (I.M. Korsunskaya, E.V. Dvoryankova, 2002) we found data on the use of a similar drug in pediatric dermatological practice. The choice of a specific drug depends on knowledge of the morphofunctional characteristics of children's skin, the mechanism of action, the chemical structure and pharmacokinetics of the drug used, local and systemic side effects. In pediatric dermatological practice, the need for prescribing glucocorticosteroids and the method of use must be clearly justified. When prescribing them, one should be guided by the following principles: maximum efficiency, safety, minimum frequency of application. Up to 40% of cases of atopic dermatitis are complicated by secondary infection of the skin (K.N. Suvorova, V.T. Kuklin, 1996), which contributes to the chronicity of allergic inflammation. To treat complicated forms of AD in children, topical corticosteroids in combination with antibiotics and antifungals are used in complex therapy.
The combination of antiallergic, antibacterial and antifungal effects in one drug makes Neoderm optimal for external treatment of complicated forms of AD in children. In order to determine the effectiveness and safety of using the drug " Neoderm " in children over 5 years of age, the drug was prescribed to 12 patients aged 6-14 years with moderate and severe atopic dermatitis, complicated by infection. The drug was prescribed 2 times a day. External therapy was combined with systemic hyposensitizing and antihistamine drugs. Regression of the clinical symptoms of the disease (reduction of itching, hyperemia, swelling, weeping) was noted already on the 2nd day of treatment in 8 out of 12 patients; on the 5-6th day of treatment, the acute inflammatory symptoms of the disease regressed in all children. The duration of external therapy in children was no more than 6 days. The data presented indicate that the use of Neoderm significantly increases the effectiveness of treatment of complicated forms of AD in children and leads to a reduction in treatment time for exacerbations.
There is also evidence of the effectiveness of the drug " Neoderm " in the treatment of pyoderma, accompanied by significant swelling, microsporia of smooth skin, mainly in cases where there was a history of atopic dermatitis of varying severity, urticaria and bronchial asthma, and there were allergic skin reactions to commonly used products. for the treatment of microsporia (iodine and sulfur-salicylic or sulfur-tar ointments). The list of indications for the use of Neoderm is constantly expanding.
In conclusion, it should be noted that the means of external treatment of allergic skin diseases have been replenished with another highly effective drug “ Neoderm ”, the use of which in both children and adults can reduce the time of treatment with combined corticosteroids and increase the effectiveness of treatment of complicated forms of allergic skin diseases.
Bibliography:
- Atopic dermatitis in children: diagnosis, treatment and prevention. Scientific and practical program of the Union of Pediatricians of Russia. International Foundation for Maternal and Child Health. Moscow.2000.-76s.
- Butov Yu.S., Lange D.A. List of antihistamines used in dermatology / Russian Journal of Skin and Venereal Diseases, 1999, No. 5.-s70-72.
- Gushchin I.S. H1 receptor antagonists as antiallergic drugs / Therapeutic archive, 1997, No. 10.-p. 27-34.
- Results and prospects for the use of topical coricosteroids from the Polish Pharmaceutical Plant Elfa A.O. Samgin M.A., Monakhov S.A. /Recipe, 2005/Appendix. - p9-16.
- Skin and venereal diseases. Guide for doctors / Ed. Skripkin Yu.K. Medicine, 1995;2:88-96.
- Kozin V.M. External pharmacotherapy of dermatoses: Textbook. Manual.-Mn.: Higher school., 1997.-80 p.
- Kozin V.M. Dermatology: Textbook.-Mn.: Higher school., 1999.-304 p.
- Korotkiy N.G., Taganov A.V., Tikhomirov A.L. Modern external therapy of dermatoses. Tver, 2001.528p.
- Medicines in Russia. Vidal Directory. M., 2005.
- Treatment of dermatoses in children. F. Zverkova, I.A. Gorlanov, V.P. Kachanov. St. Petersburg. 2nd ed., 1999.-63 p.
- External corticosteroids in the treatment of allergic dermatoses. Yu.S. Butov, Yu.A. Homeland/Recipe, 2005/Appendix.-C2-8.
- Revykina V.A. General principles of diagnosis and treatment of food allergies in children / Russian Medical Journal, 2000, volume 8, No. 7.-p.1-8.
- Sosnovsky A.T. Yagovdik N.Z. Dermatological reference book.-Mn.: Higher school, 1992.-512 p.
- I.A. Shakhtmeister, N.L. Shimanovsky ©, 1999-2000. Russian State Medical University, Moscow. Russian Children's Clinical Hospital, Moscow. Topical use of local corticosteroids.
Shimanskaya I.G.
Medical panorama No. 1, 2007.
Combined external agents containing corticosteroids
All the drugs that I mentioned above are used when dermatitis, psoriasis, eczema and other diseases proceed without any problems.
But it happens that they are complicated by a bacterial infection, and then pustules appear on standard rashes, skin hyperemia and itching intensify. In this case, 2 in 1 drugs are indicated: hormone + antibacterial component.
Such drugs include:
- Celestoderm-B with garamycin,
- Belogent,
- Diprogent,
- Hyoxyzone,
- Oxycort,
- Lorinden S,
- Flucinar N,
- Akriderm Ghent.
Hormones are also used for mycosis of the foot, if it occurs with a pronounced inflammatory reaction, when the redness of the skin, itching, and weeping appear. In this situation, a combination drug containing an antimycotic and a hormone is prescribed:
- Travocort,
- Candide B.
In difficult cases, when it is unclear what is involved here: either a bacterial or fungal infection, or all together, 3 in 1 drugs are prescribed, which contain a hormone + antibiotic + antimycotic:
- Triderm,
- Pimafukort,
- Akriderm GK.
And another group of combined drugs are drugs containing hormone and salicylic acid. In various skin diseases, for example, psoriasis, hyperkeratosis is observed. The stratum corneum thickens and external agents cannot penetrate through it. What should you do in this case? Soften it with salicylic acid.
In this situation, the best drugs are:
- Belosalik,
- Diprosalik,
- Lorinden A,
- Akriderm SK.
What should you consider when choosing a dosage form?
Now regarding the choice of dosage form.
This takes into account:
- Place of application of the drug. For example, Sinaflan, Flucinar and Fluorocort cannot be applied to the face and other delicate areas of the skin, because here the absorption of the drug is very high.
I list the absorption of external agents in different parts of the body from top to bottom from minimum to maximum:
- Sole,
- Palms,
- Forearm,
- Back,
- scalp,
- Face,
- Genitals.
- Patient's age.
The depth of penetration of the drug is maximum when used in the form of an ointment, less in the form of a cream, and even less in the form of a lotion, emulsion or solution.
Through the delicate children's skin, drugs are absorbed faster, and systemic effects will appear faster. Therefore, you must strictly adhere to the age restrictions and course duration recommended by the manufacturer. If in a particular situation both ointment and cream can be offered, it is better to give preference to the cream. But this does not mean that ointments cannot be recommended for children. With appropriate symptoms, it is possible and even necessary. We'll look into it further.
Be careful with older people too. With age, the skin loses collagen and elastin, the synthesis of skin lipids, which perform a protective function, decreases, and the epidermis becomes thinner. Therefore, if symptoms allow, it is also better for them to give preference to cream. Or those drugs that give a minimum of side effects.
- Symptoms and stage of the disease:
- Is your skin dry or not?
- Is the redness severe or moderate?
- Is the skin thickened?
- Is there any weeping?
- Are there any ulcers on the skin?
- Damage zone. The larger it is, the more topical steroid is applied to the skin, the higher the risk of systemic reactions. If both ointment and cream can be recommended due to the condition of the skin, preference is given to cream. But some modern products can be applied to large areas. Look further.
Creative strategies for topical therapy of psoriasis
In modern publications on the treatment of psoriasis, the vast majority of works belong to the study of genetically engineered biological drugs and their effect on the course and prognosis of this pathology. Despite this, the problem of optimizing topical therapy remains relevant for practicing dermatologists, since in most cases, patients suffering from psoriasis have less than 10% of the body surface affected. Low adherence to treatment, different patient preferences, different skin types and other factors require the creation of new strategies and their individual optimization for each patient.
American dermatologists from the University of California Karina Koo, Caleb Jeona and Tina Bhutania are concerned that in recent years fewer and fewer publications have been devoted to this method of treating psoriasis, which soon, given this trend, may lead to a complete loss of its use. In this regard, they performed a review of more than 15 articles related to this topic, in which they summarized the available evidence on the following five strategies for topical treatment of psoriasis:
- Proactive therapy;
- Rotation therapy;
- Sequential therapy;
- “A sharp start” with a highly active topical agent to enhance the effectiveness of drugs with a slower onset of action;
- Combination treatment using several safer, but less effective topical drugs.
Several of the above methods have been validated as treatment options for other chronic inflammatory skin diseases. However, according to the authors of this review, due to the unity of pathogenetic mechanisms, these strategies are also applicable for the treatment of psoriasis.
Preventing relapses: proactive therapy
The activity of psoriasis varies over time, its relapses are unpredictable, therefore, at the first signs of increased activity, it is possible to use safer topical corticosteroids in order to “nip in the bud” a relapse of the disease. What is proactive therapy? Its essence lies in intensive local treatment until almost complete disappearance of all lesions, followed by long-term intermittent application of topical corticosteroids in reduced doses to previously affected areas of the skin.
Since the safety spectrum of topical agents used for proactive therapy (mainly topical corticosteroids and the calcineurin inhibitor, tacrolimus) is quite favorable when used appropriately, it is necessary to ensure that these drugs are available at home in the patient so that they can be used at the first sign of an exacerbation.
The effectiveness of proactive therapy has been shown mainly for the treatment of atopic dermatitis (Wollenberg A et al., Allergy 2008; Fukuie T et al., Journal of Dermatology, 2016), but the authors of the review consider it possible to use the results of these studies in the treatment of patients with psoriasis.
Rotation therapy
Topical rotation therapy involves alternating two topical agents with different mechanisms of action to maintain the effectiveness of treatment while maintaining an acceptable safety profile with long-term use.
Typically, this strategy involves the use of steroidal and non-steroidal agents. Topical steroids are characterized by a rapid onset of action. However, it should be borne in mind that the most effective highly active topical steroids, when used for a long time, lead to the development of extremely undesirable side effects. In order to minimize such side effects when using steroids, periodic “steroid holidays” are recommended.
Rotation therapy involves an abrupt transition from a topical steroid to a safer, but less effective non-steroidal drug. This strategy can only use agents that have been shown that abrupt switching from a topical steroid will not lead to disease relapse.
The feasibility of such a strategy was explored for the clobetasol-calcitriol regimen. In a study that included 170 patients with psoriasis, they were treated with clobetasol spray twice a day for a month, followed by a sudden switch to calcitriol ointment twice a day. The results of treatment with clobetasol exceeded expectations: 93% of patients had clear, almost clear or mildly affected skin at the end of 4 weeks of therapy. Moreover, this result was maintained over 4 weeks of calcitriol therapy. A marked decrease in the proportion of patients whose disease was controlled occurred between 8 and 12 weeks of calcitriol therapy, from 92 to 74%.
The study authors concluded that the minimal risk of relapse when using the clobetasol-calcitriol regimen is achieved by switching drugs every 4 weeks, without waiting for significant deterioration. The authors of the review indicate that theoretically, such a regimen with monthly alternation of two drugs can be used for an indefinitely long time. This strategy can prevent the development of tachyphylaxis to clobetasol, which occurs with its constant use. Also, in this situation, there is no need for long-term use of steroids - “steroid holidays” are created, which are possible thanks to periods of use of calcitriol.
Thus, the proposed scheme allows for the creative use of topical agents, achieving maximum effectiveness while maintaining an acceptable level of safety in the long term. It is likely that rotation therapy can also be achieved using other means; this remains to be assessed in future studies.
Sequential therapy
Sequential therapy involves combining potent topical steroids with milder non-steroidal drugs in a predetermined sequence to maximize early efficacy and provide safer long-term maintenance therapy.
The scheme usually includes three steps:
- Induction of remission or “cleansing” phase of the skin;
- Transition phase;
- Maintenance phase.
During the first phase, the emphasis is on maximum efficiency. When you first contact a dermatologist, psoriasis is usually poorly controlled or even in a state of relapse - such a situation requires rapid resolution.
In the initial phase of treatment, daily use of highly potent topical steroids as the main agent in combination with non-steroidal drugs, such as a vitamin D analogue, as an adjuvant is possible. Clinical studies have shown that combination therapy with topical steroids and vitamin D analogues is more effective than twice daily use of high-potency steroids alone.
The second, “transitional” phase involves continuing therapy with nonsteroidal drugs twice a day on weekdays and topical steroids on weekends. The goal of the transition phase is a smooth transition from the first phase, with its emphasis on highly potent topical steroids, to the third phase of maintenance therapy, which involves the use of safer agents. The success of rotational therapy has been discussed previously, but there is no convincing evidence for the use of other highly active GCMs other than clobetasol, and therefore it is not possible to assess the risk of exacerbations or relapses when they are discontinued.
Figure 1 | Standard sequential therapy. Source: Evolution of Topical Therapy in the Treatment of Psoriasis: A Review //Journal of Psoriasis and Psoriatic Arthritis. – 2015
Figure 2 | Sequential therapy with betamethasone/calcipotriol combination. Source: Evolution of Topical Therapy in the Treatment of Psoriasis: A Review //Journal of Psoriasis and Psoriatic Arthritis. – 2015
Using a sequential strategy minimizes the risk of relapse by gradually withdrawing the highly potent steroid during the transition phase. The effectiveness of this method was demonstrated in a clinical study in which it was shown that alternating non-steroidal drugs on weekdays with steroids on weekends was twice as effective in controlling psoriasis as using steroids alone on weekends.
As part of sequential therapy, it is possible to use other regimens in which the transition from more powerful drugs to safer ones intended for long-term maintenance therapy is carried out not only within the limits of drugs for topical use, for example, a transition from cyclosporine to acitretin or phototherapy.
“Jump start” with a highly active steroid to enhance the effectiveness of drugs with a slower onset of action
Since psoriasis is a chronic disease, it is not surprising that some therapeutic strategies have been developed to favor long-term effectiveness over rapid onset of effect. This applies, in particular, to drugs for systemic use such as apremilast and acitretin, as well as to phototherapy, including UVB or a combination of psoralen and UVA. However, it is often required, based on the severity of psoriasis or the preferences of the patient, that the effect of the drug used develops in the shortest possible time. happened in an ultra-short period of time. In such situations, coadministration of high-potency steroids may hasten the onset of action of slow-acting drugs.
Therapy using a combination of safer, equally effective methods
Available drugs for the treatment of psoriasis can be divided into more effective, but less safe for long-term use, and less effective, suitable for long-term use. It is possible to use a combination of drugs from the second group in order to “summarize” their therapeutic effect while maintaining an acceptable level of safety. The rationality of this approach has been demonstrated, for example, for combinations of calcipotriol and UVB therapy; calcipotriol with systemic drugs - acitretin, cyclosporine or methotrexate.
Thus, the literature review demonstrated that many strategies for the treatment of psoriasis are based on combining drugs with different mechanisms of action. However, the review authors note that combination regimens are not rational for all drugs or treatment methods. For example, topical agents that have an acidic environment - which is the majority of topical steroids - can inactivate calcipotriol when the two agents come into direct contact. At the same time, the correct combination of certain drugs using the strategies described in the review can significantly improve the results of treatment of psoriasis.
Source:
Koo K., Jeon C., Bhutani T. Beyond monotherapy: a systematic review on creative strategies in topical therapy of psoriasis // Journal of Dermatological Treatment. – 2022. – T. 28. – No. 8. – pp. 702-708.
Algorithm for choosing the form of a topical steroid
- In case of a chronic process, which is manifested by dry skin, peeling, slight redness, ointments are prescribed.
They are distinguished by a high content of lipids, which replace the damaged lipid layer of the epidermis, for example, in atopic dermatitis. Restores skin barrier function.
Disadvantages: oily skin after application, stains on clothes.
- Creams and gels contain 2 times less lipids and do not prevent the evaporation of water from the surface of the skin. They are used for acute processes with severe redness, swelling of the skin, weeping, and also where areas of the skin come into contact with each other: inguinal folds, elbow, popliteal folds.
They are well absorbed and do not leave stains on clothes.
- Lotions, aerosols, solutions, emulsions are most often used on the scalp.
These dosage forms are also prescribed for application to the skin in the area of folds, because I already said above that the epidermis here is very thin, so a form with minimal penetration is needed to reduce the risk of unwanted reactions.
Benefits of Some Topical Corticosteroids
I will highlight several drugs that have a number of clear advantages.
Afloderm - created specifically for application to delicate areas of the skin: face, neck, genital area.
Children are allowed from 6 months, can be used by pregnant and lactating women.
Cream – for acute inflammation with weeping.
Ointment - for chronic process with dryness and flaking of the skin.
The maximum course is 3 weeks.
Advantan is a non-halogenated drug, which means it is as safe as possible. Systemic effect is minimal.
If other drugs are applied 2-3 times a day, then Advantan is applied only 1 time a day.
Can be used for a long time: in adults up to 12 weeks, in children – up to 4 weeks.
Children are allowed to use from 4 months.
The ointment is best recommended for chronic processes: dryness, slight peeling. The rashes are not bright.
Oily ointment is also used for a chronic process, but when the skin is very dry, it cracks and flakes a lot.
The cream is recommended for acute inflammation: red skin, severe itching. There are no wet areas .
Emulsion - used for acute inflammation (redness, itching, weeping ), as well as for photodermatoses, sunburn.
Advantan can be used on large areas of skin.
Does not leave greasy stains on clothes, has no odor.
Locoid is a non-halogenated, strong corticosteroid. I think you have already understood what this means: the risk of systemic effects and local adverse reactions is minimized.
Children are allowed to use from 6 months. Can be used on delicate areas of the skin.
Lokoid ointment is recommended if itching and redness are accompanied by very dry, thickened and rough skin, even cracking.
Locoid cream is used for severe skin redness and itching without weeping.
Lokoid Lipocrem is a cream rich in lipids. It is recommended if the skin is dry, flaky, and there is severe hyperemia and itching.
Lokoid Krelo - the last word means "Cream Lotion". This is a unique dosage form, which has no analogues. Used for severe inflammation and redness with weeping and blistering. Softens and soothes the skin, has a healing effect. Leaves no traces. Can be applied to the scalp and delicate areas of the skin.
Elokom is a non-fluorinated steroid with a high degree of anti-inflammatory activity. Adverse reactions are rare.
Children - from 2 years old.
It is interesting because it is applied only once a day. Can be used for a long time - up to 12 weeks.
Ointment - for dry skin.
Cream or lotion is used for inflammation of delicate areas of the skin (face, neck, folds, genitals).
The lotion is also prescribed for use on the scalp (seborrheic dermatitis, psoriasis).
If we compare Elokom with the drug Celestoderm-B, then the therapeutic effect when using it occurs 1.5-2 times faster, and it produces less side effects.
Elokom S - in addition to mometasone, contains salicylic acid, i.e. keratolytic. It is applied to rough, thickened skin. In particular, it is used for moderate and severe forms of psoriasis.
New generation topical glucocorticosteroids in external therapy of dermatoses
The effectiveness of external therapy for skin diseases is ensured by external forms of drugs successfully selected depending on the pathology and stage of inflammation. When treating skin diseases, especially those accompanied by inflammatory phenomena, it is necessary to pay attention to the fact that each stage of the disease requires different medications and methods of their use. Sometimes, despite the correctly selected medication, improvement does not occur, and then it is necessary to select a new dosage form.
Since the skin and its receptor apparatus become accustomed to the drugs, in order to achieve a therapeutic effect during the treatment process it is necessary to change the drugs. In patients suffering from allergic dermatoses (eczema, atopic dermatitis), the skin sometimes reacts with increased inflammation to substances that usually do not irritate it. Therefore, means for external treatment of this group of diseases are selected carefully. Topical glucocorticosteroids are widely used in dermatology, but sometimes their use causes complications, in particular, skin atrophy. Therefore, with great enthusiasm, dermatologists adopted a new drug - Elokom (mometasone furoate), a synthetic 17-heterocyclic corticosteroid, which began to be produced in 1987 in the USA and relatively recently (since 1996) appeared in Russia.
Clinical trials of mometasone furoate have suggested that its effectiveness is comparable to that of strong steroids. At the same time, a study of the degree of skin atrophy and side systemic effects on the pituitary-adrenal system showed that the drug is comparable in safety to weak steroids. The absorption of Elokom cream is 0.4% of the applied dose, and that of the ointment is 0.7%, which meets the criteria for an “ideal” glucocorticosteroid in terms of safety.
We studied the effectiveness of elocom (0.1% cream, 0.1% ointment and lotion). The presence of different dosage forms allows the drug to be used at different stages of the inflammatory process and at different locations (for example, on the scalp). The active ingredient is mometasone furoate, a non-fluorinated synthetic corticosteroid, which has anti-inflammatory, antipruritic and vasoconstrictor effects. All three dosage forms have a good base that maintains the pH of the skin. The high level of safety allows the use of elocom in pediatrics and geriatrics. Thus, Elokom was used with great effect in children from 6 months to 12 years old suffering from atopic dermatitis and eczema. Even after using the cream or ointment for 4 weeks, no side complications were identified, including skin atrophy. This safety in use is typical for 1% hydrocortisone ointment (with less effectiveness). A distinctive feature of Elokom is its high activity combined with the rarity of systemic (does not affect the function of the pituitary gland and adrenal glands) and local (virtually does not cause skin atrophy, hypertrichosis, folliculitis and other manifestations of secondary infection) side effects compared to other corticosteroids.
Over 3 years, under our supervision there were 285 patients suffering from various dermatoses: true eczema of the skin of the face and extremities - 129, atopic dermatitis - 98, psoriasis localized in the skin of the face and palms - 24, integumentary forms of lupus erythematosus - 16, acute allergic dermatitis — 18 who received the topical corticosteroid Elokom. Most of them were between 20 and 45 years old.
Allergic dermatitis
18 patients with acute manifestations of allergic dermatitis localized on the skin of the face were prescribed external monotherapy. Considering that the patients had acute manifestations in the form of severe swelling, hyperemia and weeping, they were prescribed Elokom in the form of 0.1% cream, which allowed for such severe In acute skin conditions, replace previously used lotions up to 15-20 times a day. The cream was applied in a thin layer to the affected areas of the skin once a day. Already on days 1-3, there was a disappearance of weeping, a sharp decrease in edema, hyperemia, and on days 4-5, complete recovery occurred, which made it possible to reduce the patients’ stay in the hospital by 2-3 times.
True eczema and atopic dermatitis
A special group consisted of 129 patients with acute and subacute manifestations of true eczema with predominant localization in the facial skin, dorsum and palmar surfaces of the hands and 98 patients with atopic dermatitis aged 18 to 37 years. The duration of the disease ranged from 12 to 30 years with frequent relapses. All patients were admitted to the clinic in the acute stage of the process. This group of patients had pronounced inflammatory changes, infiltration, lichenification in the folds, dorsum of the hands, on the neck, scattered maculopapular elements on the skin of the trunk, face, limbs, multiple excoriations, cracks, and peeling. 26% of patients with a severe recurrent course of the disease and severe itching of the skin had intolerance to many external medications (tar, naphthalan, dermatol, boric acid). As external therapy, 0.1% Elokom cream was prescribed in combination with general therapy with traditional means (vascular, sedatives, antihistamines, enterosorbents, etc.). On days 3-5, patients experienced disappearance of itching, weeping, and hyperemia. After 7-10 days, clinical recovery was noted, which made it possible to transfer patients to a day hospital to complete general therapy and reduce the treatment period to 14-20 days.
Patients with atopic dermatitis were prescribed 0.1% Elokom ointment mainly on open areas of the skin; 23% of patients received a topical steroid for the first time, and the rest were previously treated with various glucocorticoid ointments, but there was no effect recently. Elokom ointment was applied once a day in a thin layer. In 97% of patients, on days 3-5, hyperemia, infiltration, and the number of excoriations decreased and skin itching almost disappeared. The total duration of treatment with Elokom in 92 patients was 7-8 days, 4 - 10 days, in 2 people there was no effect, and Elokom was discontinued. All patients received an appropriate diet, sedation and other general therapy. The use of elocom as an external agent made it possible to transfer 98% of patients to a day hospital and reduce their stay there to 14-15 days.
Psoriasis
Patients with various forms of psoriasis were prescribed 0.1% Elokom ointment on the skin of the face and hands and 0.1% lotion on the scalp. The prescription of Elokom for psoriasis is pathogenetically justified, because it suppresses the production of three cytokines: interleukin 1, 6 and tumor necrosis factor alpha. The duration of the disease ranged from 2 to 20 years, the age of patients was from 21 to 65 years. Prescribing Elokom ointment to open areas of the skin (face, hands) and lotion to the scalp, achieving a rapid therapeutic effect already on days 5-7 allowed all patients to be transferred to a day hospital to continue general therapy and physiotherapy.
Discoid lupus erythematosus
When treating discoid lupus erythematosus, it is difficult to choose the correct external therapy that would be effective. Over the past decades, topical fluoride-containing corticosteroid ointments have been used under an occlusive dressing at night, and during the day - lubricating the lesions 2-3 times a day. Since fluoride-containing corticosteroid ointments cause skin atrophy with long-term use, their use is justified for discoid lupus erythematosus, because The ultimate goal for this disease is to obtain scar atrophy at the site of the lesion. However, one often encounters increased erythema with long-term use of fluoride-containing corticosteroids due to the formation of telangiectasia. Elokom is a fluoride-free corticosteroid drug; the determining factor for the choice of this drug was that mometasone furoate, which is part of it, has not only an anti-inflammatory, but also a vasoconstrictor effect.
We took into account not only the activity of Elokom in comparison with other corticosteroid ointments, but also the fact that Elokom does not cause the appearance of hypertrichosis or secondary infection with long-term use, which is especially important because Discoid lupus erythematosus is localized mainly on the face. External use of elocom was combined with general therapy (hydroxychloroquine, calcium supplements, nicotinic acid).
Among the patients with lupus erythematosus there were 4 men and 4 women. The duration of the disease ranged from 5 to 15 years. In all patients, the process on the skin was characterized by a severe, constantly recurrent course. Three patients had significantly hyperkeratotic changes. The ointment was applied in a thin layer once a day to the affected areas of the skin. On days 5-7, we noted a decrease in skin infiltration and the disappearance of scales; on days 14-20, erythema and hyperkeratosis disappeared, and cicatricial atrophy formed at the sites of former lesions. The therapeutic effect was not achieved in only one patient: due to increased erythema and the appearance of itching, Elokom was discontinued. In the remaining 5 patients, the drug was well tolerated and a high therapeutic effect was obtained.
Triderm
Triderm cream contains a corticosteroid (betamethasone dipropionate), an antibiotic (gentamicin) and an antimycotic (clotrimazole). Triderm was used for external therapy of 253 patients suffering from allergic dermatoses complicated by a secondary infection, as well as dermatoses resulting from a long and sluggish microbial process against the background of trophic disorders of the skin and patients suffering from chronic pyococcal dermatoses resistant to any external methods.
The first group (n=173) included patients with allergic dermatitis, atopic dermatitis, true eczema, complicated by a secondary infection. The second group (n=51) consisted of patients with paratraumatic microbial eczema, which arose against the background of a varicose symptom complex, and 15 patients with mycotic eczema, most often provoked by yeast-like fungi of the genus Candida, less often by fungi of the genus Trichophyton (red and interdigital). In addition, this group included patients with indolent chronic streptoderma - 6 people. A special group was represented by 9 patients with candidal lesions of large folds.
When prescribing the drug Triderm, first of all, its anti-inflammatory and antimicrobial (antimycotic) effects were taken into account in the absence of addiction and side effects characteristic of steroid drugs (thinning and atrophy of the epidermis, the occurrence of telangiectasia, hypertrichosis, etc.). The drug was applied in a thin layer to the lesions 2 times a day. In the majority (89%) of patients, the cream was used as monotherapy.
The most pronounced therapeutic effect was observed in patients suffering from allergic dermatitis, true and occupational eczema, complicated by secondary infection. It should be noted that the drug was applied to large surfaces of the skin, including open areas - face, neck, hands. The inflammatory reaction, manifested by hyperemia, swelling and weeping, was stopped on days 2-3, nodular rashes resolved on days 3-4 without a step-by-step selection of external agents according to the classical scheme from wet-drying dressings to epithelializing ointments, which made it possible to transfer patients after 3-5 days to follow-up treatment in a day hospital.
For patients with atopic dermatitis who had a history of long-term uncontrolled treatment with hormonal ointments, Triderm was prescribed to traditional “problem areas”, taking into account the frequent complications of the skin process in these areas by eczematization or persistent lichenification and cracks. The anti-inflammatory effect of Triderm manifested itself on days 3-7 of use, with resorption of infiltration, healing of cracks, and cessation of weeping, usually caused by secondary infection.
In the second group of patients with microbial (paratraumatic) and mycotic eczema, the prescription of triderm was aimed primarily at a microbial, often streptococcal or mycotic infection, causing a persistent allergic reaction of the skin. It should be noted that it is in these patients that the skin inflammatory process occurs against the background of severe dysfunction of peripheral vessels (varicose symptom complex, trophic ulcers, chronic thrombophlebitis, vascular obliteration, traumatic injuries, chills, etc.), so patients are necessarily prescribed general therapy ( vascular, antihistamines and other drugs).
Age-related changes, intercurrent somatic diseases, and intolerance to many medications aggravated the course of the skin process and made it difficult to choose external treatment methods. We used Triderm in this group of patients, taking into account the antimicrobial effect of its constituent gentamicin, and the antifungal effect of clotrimazole. The positive effect of the drug was manifested in a significant reduction in itching, hyperemia and swelling, drying out of oozing areas, although infiltration persisted and further required the use of absorbable ointments in combination with vascular drugs, desensitizing agents and antioxidants.
For the treatment of patients with candidal lesions of large folds, Triderm cream was used for 4-7 days only to relieve a strong inflammatory reaction, followed by a transition to other fungicidal agents. In patients with persistent lesions of the corners of the mouth caused by both candida and streptococcal infections, the drug was successfully used for 3-4 days.
Triderm was used in 3 patients with chronic benign familial pemphigus Hailey-Hailey. According to modern concepts, this is a genetically determined disease, manifested by a violation of the synthesis or maturation of keratinocyte tonofibrils, which leads to dysfunction and death of desmosomes and weakening of intercellular connections. Mechanical, chemical, as well as allergic and microbial factors contribute to the appearance of blisters on the skin in this disease. The lesions are located in the folds of the neck, axillary folds and under the mammary glands and are represented by erythematous plaques with scalloped outlines, bordered by exfoliating epidermis. The surface of the plaques was macerated and covered with tortuous, medullary fissures separating serous-purulent exudate; an accumulation of yellowish crusts was noted at the periphery of the lesions.
Triderm cream was applied 2 times a day for 7-19 days. The effect in the form of flattening of the affected areas occurred by 4-5 days. It is important to note that persistent epithelization of erosions occurred, the formation of fresh blisters ceased, the surface was cleared of cortical layers, which made it possible to achieve remission lasting from 6 months to 1 year, the relapse of the disease was much weaker than before using the drug. The use of Triderm monotherapy for benign familial pemphigus made it possible not to prescribe oral glucocorticoids.
conclusions
The existence of 3 dosage forms of Elokom - lotion, cream and ointment - makes it possible to use it at different stages of the inflammatory process. Elokom has a pronounced anti-inflammatory, vasoconstrictor and especially antipruritic effect, which makes it possible to use it in the form of a 0.1% cream for acute skin conditions. Using elocom once a day is convenient and cost-effective. The virtual absence of side effects allows you to use Elokom even when the inflammatory process is localized on the skin of the face. Elokom quickly stops the clinical manifestations of the disease and allows patients to be transferred to treatment in a day hospital, which significantly reduces the duration and cost of hospitalization and improves the quality of life of patients. The use of elocom in children for the relief of acute and chronic dermatoses is possible from the age of 2 years.
Triderm is a fast-acting drug with a wide spectrum of action, which allows it to be used in the treatment of dermatoses of an allergic, bacterial and mycotic nature, as well as torpid dermatosis of hereditary origin (Hailey-Hailey disease).
Betamethasone + clotrimazole + gentamicin –
Triderm (trade name)
(Shering-Plough)
Mometasone furoate –
Elokom (trade name)
(Shering-Plough)
| Applications to the article |
“How much to weigh in grams”? Dose at your fingertip
Have your customers ever asked you how much ointment should be squeezed out of a tube? After all, this also affects the therapeutic effectiveness and the risk of side effects. If you apply a little, it will not work, or it will have a weak effect. If you apply a lot, there may be a burning sensation, redness and other undesirable reactions.
Some good person came up with a unit of measurement for an external product.
It's called a fingertip unit, or FTU.
The amount of the drug squeezed out of a tube with a hole with a diameter of 5 mm and occupying the distance from the tip to the nearest joint on the index finger on the palm side is one dose. One CCP is approximately 0.5 grams.
See:
To help you figure out how much of the external product goes to different parts of the body, and when to offer what volume of tube, I found this wonderful diagram:
At the end of this conversation, I would like to summarize:
When choosing an external remedy, do not forget to find out who is taking it, what part of the body they are going to smear, what is the area of the affected area, and also:
- Is your skin dry or not?
- Is the redness severe or moderate?
- Is the skin thickened?
- Is there any weeping?
- Are there any ulcers on the skin?