Among the many medications used for acne are a group of retinoids, derivatives of vitamin A.
Although topical use of these agents is quite common, with dry skin being the main side effect, their oral use carries a risk of fetal harm if pregnancy occurs during or in the short term after completion of treatment.
Deterioration of liver and lipid parameters of the body is also often observed. Therefore, oral retinoid therapy is reserved exclusively for the most severe forms of acne.
What is isotretinoin
Isotretinoin is an organic chemical compound and a synthetic derivative of vitamin A, which is a stereoisomer of all-trans retinoic acid (tretinoin). It is a potent substance used primarily in the treatment of all types of acne, including nodular cystic acne and pimples.
In particular, isotretinoin is used to combat severe forms of the disease that are resistant to standard treatments. The effect of the drug is to stop the growth of the sebaceous glands and reduce their activity. In small doses, dermatologists successfully treat other skin diseases with it - seborrhea, seborrheic dermatitis and rosacea. Retinoids also provide excellent results in anti-aging treatments.
Clinical studies have shown that this substance is very effective in treating acne. It is ideal for cases where antibiotics or other topical medications do not help. It has also been found that in addition to reducing the activity of the sebaceous glands of the skin (up to 80%), isotretinoin causes a reduction in their size by 35-58%, stimulates the renewal of epidermal cells and reduces their keratinization. The drug also has an anti-inflammatory effect.
People struggling with severe acne write rave reviews about the results of using the drug on online forums. Spectacular photographs taken before and after treatment are posted on social networks. The prospect of getting rid of annoying defects that significantly spoil your mood is tempting. However, before considering such treatment, it is worth learning more about it.
Why does acne occur?
The content of the article
Acne is a group of diseases associated with disorders of the sebaceous glands. The most popular form of acne appears to be acne vulgaris, which usually affects teenagers and some adults. As a result of hormonal changes, the sebaceous glands produce excessive sebum. In addition, we often encounter increased keratosis of cells that clog the excretory ducts, which contributes to the formation of acne and purulent pustules.
Fact 1. Treatment of acne with isotretinoin requires close supervision by a dermatologist.
The drug is available by prescription and should be used under the supervision of an experienced dermatologist. Isotretinoin may not cure acne, but may harm your health. The dermatologist individually selects the appropriate form and dose of the medicine for each patient, calculates the frequency of administration and duration of treatment.
In the treatment of milder acne, isotretinoin is used in lower doses, and in cases with complex acne, including genetic background, doses with higher concentrations are recommended. Also, the dose of isotretinoin is adjusted depending on body weight, type of acne, location (only the face, as well as décolleté and back), severity and depth of scars.
Isotretinoin for acne can be used in different forms - orally and topically. When treating severe forms of acne, ointment is used, since it contains the maximum permissible concentration of the active substance, due to which it shows the highest intensity of action. Gels are recommended for milder forms of acne. They have a light texture and are quickly absorbed through the skin. The drug in the form of a cream contains the lowest concentration of the active substance and is most often used to maintain the effects of treatment.
The course of treatment lasts from 16 to 24 weeks. Each patient is treated individually, so don't ask your friends what dosage they are taking.
After the first course of treatment, its effectiveness is assessed. If therapy is unsuccessful, therapy can only be resumed after 8 weeks. For treatment to be effective and safe, it is imperative to follow the recommendations of your doctor.
The use of isotretinoin for the correction of wrinkles –
But the retinoid isotretinoin can be used not only systemically (orally).
There are a small number of drugs with isotretinoin for external use, which were initially used exclusively for the treatment of acne and pimples, but later these drugs began to be used to correct the symptoms of photoaging. As in the case of the retinoid tretinoin, preparations with isotretinoin also help to increase skin elasticity and reduce the depth of wrinkles. What effects do external forms of isotretinoin cause in the skin:
- Peeling effect - the thickness of the superficial stratum corneum of the epidermis decreases (due to exfoliation of dead skin cells). This evens out skin tone and texture, leaving skin looking more youthful and radiant—like you've had a few superficial chemical peels.
- Increasing the thickness of the deep layers of the epidermis - isotretinoin affects stem keratinocytes located at the basement membrane, increasing the rate of their division and differentiation. This leads to an increase in the thickness of the deep layers of the epidermis, consisting of living keratinocytes. As a result, the hydrophobicity of the epidermis increases, which contributes to less evaporation of moisture from the surface of the skin. In addition, it prevents skin photoaging.
- Stimulation of the production of collagen and hyaluronic acid - isotretinoin affects not only the epidermis, but also the dermis. It promotes the proliferation (reproduction) of fibroblasts, and also significantly stimulates their activity, which leads to an increase in their production of collagen, elastin and endogenous hyaluronic acid. This leads to an increase in the thickness of the dermis, a decrease in the depth of wrinkles, and an increase in skin elasticity. It is known that thicker skin is less susceptible to the aging process.
Optimal concentrations of external forms of Isotretinoin –
There are a number of clinical studies where scientists have determined the optimal concentration of isotretinoin for the treatment of photoaging.
1) “Armstrong RB, Lesiewicz J, Harvey G et al. Clinical panel assessment of photodamaged skin treated with isotretinoin using photographs. Arch Dermatol 1992; 128:352–6.” 2) “Sendagorta E, Lesiewicz J, Armstrong RB. Topical isotretinoin for photodamaged skin. J Am Acad Dermatol 1992; 27:S15–18.”
In these studies, the concentration of Isotretinoin was increased from 0.05% at the beginning of the study to 0.1% at the end of the study. Despite the increase in concentration, the drug was well tolerated by patients without causing significant skin irritation. As a result of the treatment of photoaging skin with 0.1% Isotretinoin, the skin condition gradually improved throughout the 36-week treatment, and a decrease in the depth of wrinkles and fine lines was achieved.
3) The study “Maddin S, Lauharanta J, Agache P et al. Isotretinoin improves the appearance of photodamaged skin: results of a 36-week, multicenter, double-blind, placebo-controlled trial. J Am Acad Dermatol 2000; 42:56–63.” In this study, a combination of 0.05% Isotretinoin plus SPF sunscreen was used to treat photoaging. The result was that the condition of skin with visible photodamage was significantly improved, which was recorded using profilometry.
Conclusions: if you are interested in preventing skin photoaging, it is best to use a 0.05% concentration in combination with sunscreen. If you want to achieve an increase in skin elasticity and a decrease in the depth of wrinkles, then the main treatment should be carried out using a 0.1% concentration (it is better to use a 0.05% concentration for the first month so that the skin gets used to retinoids).
Isotretinoin has a delayed effect - it will take at least 8-12 weeks before you notice any positive changes, although the first positive effect associated with improved skin tone and texture will be noticeable after 4-6 weeks. It must be admitted that abroad isotretinoin is used for the correction of photoaging much less frequently than other types of retinoids - tretinoin or pure retinol.
Preparations with isotretinoin for external use –
- “Retinoic ointment” (Fig. 8) – is available with an isotretinoin concentration of 0.05% or 0.1%. The cost will be from 300 rubles for a 15 g tube. About a tenth of the volume is ethyl alcohol, so you should not use this drug if you have dry and/or sensitive skin. In principle, the manufacturer writes in this regard that this drug is intended for the treatment of acne in patients with oily skin.
- "Retasol" (Fig. 9) - is a solution for external use, with an isotretinoin concentration of 0.025%. But keep in mind that this drug also contains alcohol, but in less quantity than retinoic ointment. In addition, the drug contains propylene glycol, which may cause irritation in patients with sensitive skin. Cost from 400 rubles per 50 ml bottle.
Fact 2. Isotretinoin is prescribed when other medications do not work.
Isotretinoin is a first-line drug only for more severe forms of acne:
- purulent;
- knotty;
- cystic;
- scarring.
Currently, indications are also extended to all forms of acne that cannot be treated with other methods.
For diseases of mild to moderate severity, for example, comedones, firstly, external treatment and oral antibiotics are used. And only in case of relapse can you resort to isotretinoin.
Acne Treatment with Isotretinoin Photo Licensed by Envato Elements Item
Fact 4. The drug has side effects
Side effects may occur when using the drug in tablet form and externally in the form of an ointment, cream or gel.
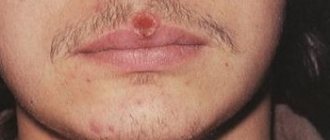
Mild side effects include drying of the mucous membranes of the nose and throat, which can lead to bleeding, dry eyes, conjunctivitis and sore lips. The severity of such side effects depends mainly on the dose used and usually disappears when the dose is reduced. Some symptoms, however, may persist even after the end of therapy.
Severe complications may also occur during treatment:
- dizziness and headaches;
- drowsiness;
- pain in joints and muscles;
- increased levels of cholesterol and glucose in the blood;
- alopecia;
- hematuria and proteinuria.
Due to the risk of twilight vision impairment, extreme caution should be taken when driving at night.
To avoid side effects, you should inform your doctor before starting treatment about the following points:
- About all medications you are taking – in particular, tetracycline and medications containing vitamin A.
- About concomitant diseases, such as:
- liver failure;
- dry eye syndrome;
- increased blood lipid levels;
- vitamin A hypervitaminosis;
- asthma;
- heart diseases;
- diabetes;
- eating disorders;
- menstrual irregularities;
- problems with the skeletal system;
- problems with the digestive system;
- depression.
3. About pregnancy, breastfeeding and contraception.
Fact 5. When treated with isotretinoin, forget about pregnancy
The main contraindications to the use of isotretinoin are pregnancy and lactation. Isotretinoin has the ability to bind 99.9% to plasma proteins, therefore it easily penetrates the placenta and breast milk, and is also present in the hepato-intestinal circulation.
The use of isotretinoin during pregnancy is very dangerous, which is associated with a strong teratogenic effect. The drug can cause miscarriages and severe fetal malformations. Therefore, when treating acne with isotretinoin, it is necessary to use an effective method of contraception one month before and for two months after stopping treatment.
During treatment and for one month after its completion, you should not donate blood, as there is a risk of fetal harm in pregnant women who receive blood transfusions containing isotretinoin.
The introduction of oral isotretinoin into medical practice has brought about a revolution in the treatment of acne. This drug made it possible to successfully treat severe forms of nodular cystic and other acne that were not amenable to previously existing treatment methods.
In recent years, there has been a trend toward increased use of isotretinoin. It has proven effective not only for acne, but also for many other skin diseases. Indications for the use of the drug that are not officially recognized include hidradenitis suppurativa, ichthyosis, keratosis pilaris (Darier's disease), lichen planus, pityriasis versicolor pilaris, lupus erythematosus (cutaneous forms), acne rosacea, mycosis fungoides (T-cell lymphoma of the skin) . It is also prescribed prophylactically for nevoid basal cell carcinoma and for the prevention of skin cancer in patients with xeroderma pigmentosum. Isotretinoin is also used to treat cancer. It is effective as monotherapy, but, according to the results of most studies, the best effect is obtained when it is used simultaneously with other drugs, in particular with cytokines. In experimental studies, oral retinoids have been studied in myeloid leukemia, squamous cell carcinoma of the head and neck, breast cancer, cervical intraepithelial dysplasia, and cervical and kidney cancers. The number of new patients taking isotretinoin is constantly increasing. In the USA alone it amounts to 500 thousand people per year [1]. In the period from 1992 to 2000. consumption of isotretinoin in the country increased 2.5 times [2]. In 2000, the drug was prescribed to approximately 2 million patients. There is a trend towards increased use of isotretinoin for mild and moderate forms of acne: in the period from 1993 to 2000. the proportion of its prescriptions for severe acne decreased from 63% to 46%, while for mild and moderate acne it increased from 31% to 49% [2].
However, although the effectiveness of isotretinoin has been convincingly proven in clinical studies and widespread medical practice, its use is limited by side effects. At least 50 acute and chronic adverse reactions that occur during treatment with the drug have been described. Of greatest concern are teratogenic and central side effects.
Teratogenic effect and precautions during pregnancy
The ability of isotretinoin to cause severe congenital anomalies was first described in 1983, i.e. one year after its approval for marketing in the United States [3,4]. By this time, a teratogenic effect had been identified in other retinoic acid derivatives, so it was also expected for isotretinoin. Since its approval for medical use, the drug has been assigned an FDA category "X", which includes drugs that are absolutely prohibited during pregnancy, since the risk of their use outweighs any potential benefit.
Isotretinoin crosses the placenta relatively easily. Its teratogenic effect is associated with the slow elimination of the 13-cis isomer, the formation of 4-oxo derivatives during metabolism, ongoing isomerization, significant exposure of target tissues to trans-retinoic acid and insufficient interaction with cytoplasmic binding sites, which leads to higher intake drug into cell nuclei [7]. Its metabolite 4-oxoisotretinoin accumulates in the fetal liver, and small concentrations of retinoids are also found in the brain [5].
Various congenital disorders have been described in children whose mothers took isotretinoin during pregnancy, including pathology of the skull; ear lesions (reduction in size or absence of the auricle, narrowing or absence of the external auditory canal); eye lesions, including microophthalmia; violation of facial morphology; cleft palate; damage to the central nervous system, incl. abnormalities of the cerebral cortex, cerebellar pathology, hydrocephalus, microcephaly, hypoplasia of cranial nerves; cardiovascular abnormalities; pathology of the thymus and parathyroid glands [6,7] Cases of shortening of the limbs, similar to those with intrauterine exposure to thalidomide, have been described [8]. It is believed that birth defects are the result of disturbances in the morphology and motility of neural crest cells during a critical stage of fetal development [9]. In many cases, the anomalies are incompatible with life and lead to intrauterine death.
The teratogenic potential of the drug is quite high and, according to various studies, ranges from 15% to 45%, which is comparable to that for thalidomide [6,10]. Between 1982 and 1989 The manufacturer of isotretinoin received 151 reports of full-term pregnancy outcomes in women treated with isotretinoin. Moreover, congenital pathology was observed in 47% of cases.
The maximum risk of developing congenital anomalies is observed when isotretinoin is used in the first trimester of pregnancy, and they are registered even in the case of taking one capsule of the drug [11]. The frequency of spontaneous abortions during therapy is 20-30% [10]. The rate of induced abortion in women taking oral retinoids, according to an analysis conducted in Australia, was 1 in 319 courses of treatment [12]. Isotretinoin also increases the risk of preterm birth. A number of children exposed to the drug in utero showed a delay in the level of intellectual development according to the IQ test.
In July 1988, the United States began training pregnant women in the use of isotretinoin as part of the Pregnancy Prevention Program [13]. The program included the distribution of printed materials for patients, instructions for physicians on scheduling pregnancy tests, and information about enrolling patients in follow-up care. Women taking part in the program were required to sign a consent form indicating that they had been counseled. The implementation of the program led to significant positive results, but did not achieve the goal of zero pregnancy rates [13,14].
A survey of women participating in the Pregnancy Prevention Program in 1989-1993 showed that almost all of them were aware of the harm that isotretinoin can cause the developing fetus [13]. Among sexually active women, 99% reported using contraception. Despite this, 3.4 pregnancies have been documented for every 1000 treatments. Approximately 75% of registered pregnancies were terminated, and 32 resulted in live births. Among the 30 children about whom information was available, developmental anomalies were noted in 7.
In a more recent study from California, 23 pregnancies were reported over a 2-year period [15]. Information about their outcomes was available in 14 cases. Of these, in 4 cases there was a miscarriage, 5 pregnancies were terminated, one newborn had a congenital pathology, 4 children were born healthy.
Study results indicate that 14-33% of women who reported pregnancy during treatment did not undergo pregnancy testing or did not wait for test results and were pregnant before isotretinoin was prescribed, and 12-16% of women became pregnant during the period after initiation of treatment. but before the onset of the next menstruation [16,17]. In addition, approximately 64% of women were unable to avoid behaviors associated with a high risk of conception [17]. In most cases, pregnancy occurred in those who used one method of contraception.
The total number of pregnancies occurring during isotretinoin treatment remains unknown. In September 2000, the drug's manufacturer reported 1,995 cases to the FDA [18]. It is believed that this figure, although unexpectedly high, is actually an underestimate due to the voluntary nature of the Pregnancy Prevention program. It is estimated that only 40% of women taking isotretinoin during pregnancy participate in the program [15]. Currently, the number of pregnancies occurring during treatment appears to be increasing due to the increase in the number of women taking the drug for various indications.
In May 2000, it voluntarily made significant changes to the instructions for use of isotretinoin. A number of the most serious warnings are placed in a frame (the so-called “black box”) to attract the attention of consumers. They say that women should:
- use the drug only in the presence of severe acne that is resistant to other types of treatment;
- understand the instructions for using isotretinoin well;
- perform all pregnancy tests;
- receive oral and written warnings about the risk of teratogenicity;
- receive oral and written information about the need to use two types of contraception during treatment;
- have negative pregnancy test results at the first determination and a second determination performed on the 2nd day after the next menstrual period, or 11 days after the last sexual intercourse;
- receive information about the opportunity to participate in the isotretinoin research program and view the manufacturer's videotape.
Due to the long half-life of the drug, measures to prevent pregnancy should be observed for a month after stopping treatment. Later after discontinuation of isotretinoin, the incidence of congenital anomalies, spontaneous and medical abortions does not differ significantly from those for the general population [19].
Women taking isotretinoin should not donate blood during treatment and for one month after its cessation, since transfusion of their blood to pregnant women carries a risk to the recipient's fetus [3].
The high lipophilicity of isotretionine determines its penetration into breast milk and the risk of adverse effects on the child.
Side effects from the central nervous system and sensory organs
In recent years, the central side effects of isotretinoin—depression and associated suicide—have been of particular concern [20]. A possible link between isotretinoin use and depression or suicide was suspected many years ago. Psychiatric side effects began to be reported to the FDA shortly after the drug entered the pharmaceutical market [21]. Between 1982 and 2000 The Agency received 431 reports of depression, suicidal motivation, suicide attempts, or suicide in patients receiving isotretinoin [22]. Among the suicides (37 cases), the vast majority (84%) were men, with an average age of 17 years. Eight of them had a history of mental illness [22].
Depression was included as a side effect of the drug in 1985. In February 1988, the FDA added a black box warning for psychiatric disorders, including suicide [18]. However, the role of isotretinoin in the development of mental disorders remains unproven.
It is known that approximately 30-50% of people aged 12-20 years experience psychological reactions due to acne, ranging from mild anxiety to severe depression, decreased self-confidence and self-esteem, and impaired social adaptation. In most patients, successful treatment of the underlying disease also leads to an improvement in psychosocial condition. However, in some patients, depression develops during the treatment period and even several months after its completion, which makes it possible to suspect that they have other problems that they previously associated exclusively with their appearance.
Concern among physicians and patients about the association between isotretinoin and adverse psychiatric reactions, supported by the media, prompted the manufacturer to conduct a special population-based cohort study that analyzed information contained in the Canadian Saskatchewan Health and Research Database. UK General Practice Database [23]. In total, the study included data from about 8,000 patients treated with isotretinoin and more than 14,000 controls treated with antibiotics. The study results do not suggest an increased risk of depression, suicide or other mental disorders with isotretinoin use. FDA officials came to the same conclusion after analyzing all available data [22,24]. Similar results were recently obtained in Australia [25].
To further investigate the potential risk of psychiatric side effects, in 2001 the manufacturer developed a form that asks patients to indicate symptoms of depression or psychosis in their immediate family or themselves prior to starting treatment with isotretinoin. In addition, it emphasizes the need to promptly report to the physician all psychiatric symptoms that occur during treatment [1,18].
A prospective analysis of side effects in 124 patients treated with isotretinoin at a dose of 1 mg/kg for 4 months showed that depression was observed in 4% of cases and tended to persist throughout the course of therapy [26].
A special prospective comparison study of antibiotics and topical agents, including 215 participants (mean age 20 years), failed to detect a statistically significant difference between groups in depression scores (p = 0.62) and quality of life [27]. There was also no correlation shown between the dose of isotretinoin and the severity of depression. Obviously, larger studies are needed to make a final conclusion about the absence of a cause-and-effect relationship between the use of this drug and depression.
In 2002, the pharmaceutical company sent US doctors a “Dear Doctor” letter, notifying them of changes in the instructions for use of the drug [29]. They included warnings about the potential for aggressive and violent behavior under the influence of isotretinoin and information for doctors and patients based on the results of a new study in pediatrics. It showed that 29% of children treated with isotretinoin experienced back pain, and 22% developed atralgias. In addition, two potentially adverse drug interactions were added to the instructions: with systemic corticosteroids and phenytoin.
In addition to depression, isotretinoin can cause other central nervous system symptoms. These include: insomnia or pathological drowsiness, fatigue, excitability, loss of appetite and interest in work or study.
In rare cases, isotretinoin (mainly when combined with tetracyclines) can cause pseudotumor cerebri. Due to the risk of increasing intracranial pressure, tetracyclines should not be taken concomitantly with isotretinoin [27]. Patients should be warned about the symptoms of pseudotumor - severe headache, nausea, vomiting and visual disturbances [1].
An analysis of 1741 reports containing information about ophthalmic adverse reactions of isotretinoin revealed 38 different symptoms of eye damage [30]. The most common among them were dry eyes (40%), blurred vision (including twilight vision), photophobia, blepharoconjunctivitis, discomfort in the eye, clouding of the cornea, increased sensitivity to contact lenses, keratitis, increased osmotic pressure of tear fluid, development of cataracts and congenital eye pathology [26,31]. Ophthalmic side effects can develop within a few days or several years after starting to use the drug. In a special study that included 40 patients receiving isotretinoin at a dose of 0.5-1 mg/kg/day for 2 months, 42.5% of participants had conjunctival colonization with Staphylococcus aureus [32]. However, this study also showed that when the drug is used in low doses for short courses, visual impairment is usually not serious and completely disappears within a month after the end of therapy [32].
Cases of hearing loss have also been reported in patients receiving isotretinoin [33].
Other adverse reactions
The most common side effects of oral isotretinoin, occurring in more than 90% of patients, are cheilitis or xerosis of varying severity. Skin adverse reactions also include dermatitis (50%), itching (
In a number of patients, during treatment with isotretinoin, carbohydrate metabolism is disrupted [26,33]. In case of an overdose of the drug, hypervitaminosis A may develop [38].
Recommendations for the correct use of isotretinoin
Despite the huge number of potential side effects of isotretinoin, practice shows that when used correctly it is a highly effective and fairly safe drug [39]. Before prescribing a drug, it is necessary, first of all, to weigh the benefit/risk ratio for a particular patient. Its use should be considered in severe forms of acne or in cases where acne is associated with severe psychosocial disorders. The use of the drug for milder forms of acne should be resorted to only in cases where repeated long courses of antibacterial therapy have not brought the desired result, when relapses develop after several successful courses of treatment with standard means, in patients with a tendency to form scars.
It should be remembered that absolute contraindications to the use of the drug are pregnancy, breastfeeding, hypervitaminosis A, and hypersensitivity. Treatment with isotretinoin is not recommended for persons with hepatic and renal insufficiency, diabetes mellitus and hyperlipidemia. Relative contraindications for taking retinoids are also pseudotumor cerebri, inflammatory bowel disease, hepatitis and childhood.
Women of childbearing age should be given detailed instructions about the risk and frequency of teratogenic effects and asked to sign a form confirming that they understand the risk of conception during treatment before being prescribed the drug. The drug is prescribed only after receiving a negative pregnancy test result and against the background of effective contraception using two methods. Since androgens play an important role in the pathogenesis of acne, when treating with isotretinoin, contraceptives containing a progestin with androgenic action, for example, 19-nortestosterone derivatives, should not be used. It is advisable to start treatment on the second or third day of the next menstrual cycle. The pregnancy test should be repeated 4 weeks after the end of therapy. The drug must be taken under the close supervision of a specialist. Clinicians who have not received specific training in the use and monitoring of oral retinoid therapy should not prescribe these drugs.
Most of the side effects of isotretinoin are dose-dependent, so it should be used in the minimum effective dose, however, to reduce the likelihood of relapse, the course dose of the drug should reach at least 120 g/kg. The recommended initial dose is 0.5-1 mg/kg per day. It should be divided into 2 doses. The dose can be increased to 2 mg/kg/day. During the treatment period, it is recommended to determine the level of liver enzymes, triglycerides and cholesterol. A significant increase in triglyceride concentrations in some cases led to the need to discontinue treatment. However, an analysis of data on 907 patients receiving isotretinoin for 5-9 months showed that triglyceridemia above 400 mg% is quite rare - in 1.5% of patients [40]. No pronounced increase in liver enzyme activity leading to drug discontinuation was observed in patients included in the analysis. The data obtained allowed the authors of the analysis to suggest that routine laboratory monitoring (with the exception of pregnancy tests) is not indicated for healthy young patients.
It should be remembered that isotretinoin has clinically significant interactions with food and drugs. Under the influence of food, its bioavailability increases by 2 times. Taking the drug with food reduces fluctuations in bioavailability. Simultaneous use with isotretinoin of other drugs for the treatment of acne - drugs with keratolytic or exfoliative effects, as well as ultraviolet therapy is not recommended. Patients should avoid other types of ultraviolet radiation, such as insolation. During the treatment period and for six months after its completion, due to the risk of developing dermatitis, hair removal using wax applications is not recommended.
Isotretinoin should not be co-administered with other vitamin A derivatives (risk of exacerbating symptoms of hypervitaminosis A). As mentioned above, the combined use of the drug with tetracyclines, corticosteroids and phenytoin should be avoided.
If the above recommendations are followed, the benefits of using isotretinoin in most cases significantly outweigh the risks [40]. The main reasons for the development of side effects of isotretinoin and unplanned pregnancies during the treatment period are non-compliance with these recommendations by medical professionals and violation of precautions to prevent conception by patients [41,42]. For example, in the United States, at least 47% and likely 72% of patients do not receive standard therapy (eg, a topical retinoid or oral antibiotic) before isotretinoin initiation [41]. Doctors often ignore the possibility of pregnancy in girls under 16 years of age, and therefore patients in this age group often do not receive the necessary consultations [42]. According to foreign studies, no more than half of doctors perform a pregnancy test before prescribing the drug, and in cases where such a test is performed, preference is given to a less sensitive urine test [43]. A study from the Motherisk program in Canada found that distributing educational materials to patients significantly improved contraceptive adherence [44]. If conception does occur during the treatment period, women should be asked to consider terminating the pregnancy, again explaining the potential danger to the fetus.
One of the latest measures taken by the FDA to improve the safety of isotretinoin use was the introduction at the end of 2002 of restrictions on the import into the country of 10 potentially toxic prescription drugs, the use of which requires special control [45]. Persons attempting to bring these drugs into the United States are denied entry. This list also includes isotretinoin.
In addition, a new microionized dosage form of the drug has now been developed, which, having a profile of adverse reactions similar to standard isotretinoin, is less likely to cause some adverse effects, for example, lesions of the skin and mucous membranes, hypertriglyceridemia [46].
Fact 6: Isotretinoin treatment requires special care
When treating acne with isotretinoin, the following symptoms are very often observed:
- dry lips;
- cracking of the corners of the mouth;
- drying of the mucous membranes of the nose, mouth and eyes;
- conjunctivitis;
- nosebleeds;
- dry skin of the face and hands.
Also, there are patients who do not feel any discomfort other than chapped lips.
Dry skin can be soothed with cosmetics and moisturizing dermocosmetics for sensitive skin. Do not use heavy, greasy creams or preparations with vitamin A. Regular moisturizing of the skin will reduce itching.
Since the skin weakens and thins during the procedure, it should not be scratched. This can lead to irritation and red spots. Lips need to be lubricated several times a day to prevent them from cracking. If necessary, you can use moisturizing eye drops.
During treatment and for another 6 months after acne treatment, avoid waxing and strong abrasion of the epidermis. You should also not use anti-acne products (gels, creams, masks, foundations). This can make dry skin worse. Also, you should avoid exfoliating peels using acids and laser procedures.
You should also protect yourself from the sun, as retinoids make the skin more sensitive to ultraviolet radiation. There is no benefit to stopping treatment because the acne will get worse after the holiday. In summer you can take isotretinoin, but you should always protect your skin with a strong sunscreen, minimum SPF 30.
Forms of acne treatment
There are many forms of acne therapy available today, although it often takes some time to find one that's right for you. The first step is to take appropriate skin care with products designed for the skin of people struggling with this problem.
Another treatment for acne is primarily the disinfectant benzoyl peroxide, which inhibits keratosis cells with azelaic acid or antibiotics.
A special group of drugs consists of retinoids, and among them isotretinoin. Although topical treatment with these agents is quite common, oral administration (due to numerous side effects) is reserved exclusively for the most severe cases of acne, such as comedones, pimples, purulent fistulas and acne scars.
Fact 7. There is no guarantee that acne will not return
The effect of the treatment can be seen after a few weeks of taking the pills, and not only on the face. Since the sebaceous glands work much more slowly, the oiliness in the hair disappears first.
Isotretinoin therapy always brings results. However, the drug does not guarantee the absence of relapse. They are, of course, much less common than after treatment with antibiotics, but they still happen.
The next treatment can begin no earlier than 8 weeks after the end of the previous one. Your doctor will usually prescribe a topical medication or a small dose of isotretinoin.