Keppra, 1 piece, 300 ml, 100 mg/ml, oral solution
IV
drip, in the form of infusion, for 15 minutes.
The transition from oral to intravenous use and back can be carried out while maintaining the dose and frequency of administration.
One bottle of concentrate for solution for infusion contains 500 mg of levetiracetam (100 mg/ml).
The daily dose is divided into two injections of the same dose.
Before use, the concentrate must be diluted with a solvent of at least 100 ml.
Instructions for dosing the solution
| Dose, mg | Volume of the drug | Solvent volume, ml | Infusion time, min | Administration frequency, once a day | Daily dose, mg/day |
| 250 | 2.5 ml (half ampoule 5 ml) | 100 | 15 | 2 | 500 |
| 500 | 5 ml (1 ampoule of 5 ml) | 100 | 15 | 2 | 1000 |
| 1000 | 10 ml (2 ampoules of 5 ml) | 100 | 15 | 2 | 2000 |
| 1500 | 15 ml (3 ampoules of 5 ml) | 100 | 15 | 2 | 3000 |
The following solvents can be used:
— 0.9% sodium chloride solution for injection;
— lactated Ringer's solution for injection;
— 5% dextrose solution for injection.
The solution remains chemically stable at 15–25 °C for 24 hours in PVC bags.
However, from the point of view of microbiological purity, the drug must be used immediately after dilution.
If necessary, the solution can be stored at a temperature of 2 to 8 °C for 24 hours, provided that the dilution was carried out under aseptic conditions. In this case, responsibility for microbiological cleanliness lies with the user.
The use of the drug is not allowed if the color of the solution changes or mechanical inclusions appear.
Monotherapy
Adults and teenagers over 16 years of age.
Treatment should begin with a daily dose of 500 mg, divided into 2 administrations (250 mg 2 times a day). After 2 weeks, the dose can be increased to the initial therapeutic dose of 1000 mg (500 mg 2 times a day). The maximum daily dose is 3000 mg (1500 mg 2 times a day).
As part of complex therapy
Children from 4 to 11 years old and adolescents 12–17 years old weighing up to 50 kg.
Treatment should begin with a daily dose of 20 mg/kg, divided into 2 administrations (10 mg/kg 2 times a day). The dose can be changed by 10 mg/kg every 2 weeks until the recommended daily dose is reached - 60 mg/kg (30 mg/kg 2 times a day). If the recommended daily dose is not tolerated, it may be reduced.
There is no clinical experience with the infusion of levetiracetam for periods exceeding 4 days.
The minimum effective dose should be used.
Recommended dosages for children (from 4 years old) and adolescents (up to 17 years old)
| Body weight, kg | Initial dose: 10 mg/kg, 2 times a day | Maximum dose - 30 mg/kg, 2 times a day |
| 15 | 150 mg 2 times a day | 450 mg 2 times a day |
| 20 | 200 mg 2 times a day | 600 mg 2 times a day |
| 25 | 250 mg 2 times a day | 750 mg 2 times a day |
| from 50 | 500 mg 2 times a day | 1500 mg 2 times a day |
Adults and adolescents weighing more than 50 kg.
Treatment should begin with a daily dose of 1000 mg, divided into 2 administrations (500 mg 2 times a day). Depending on the clinical response and tolerability of the drug, the daily dose can be increased to a maximum of 3000 mg (1500 mg 2 times a day). The dose can be changed to 500 mg 2 times a day every 2–4 weeks.
The duration of the course of treatment is determined by the doctor.
Inside,
regardless of food intake. The daily dose is divided into 2 doses of the same dose.
The tablets are taken with a sufficient amount of liquid.
The tablets are not intended for children under 6 years of age due to the impossibility of selecting the correct dose.
In the case of a solution, dosing is carried out using a measuring syringe included with the drug, with a nominal capacity of 10 ml (corresponding to 1000 mg of levetiracetam) with a division value of 25 mg (corresponding to 0.25 ml). The measured dose of the drug is diluted in a glass of water (200 ml).
Dosing of the solution is carried out using measuring syringes included in the delivery package of the drug. Syringes are available with nominal capacities:
- 10 ml (corresponding to 1000 mg of levetiracetam) and with a division price of 0.25 ml (corresponding to 25 mg) for children 4 years and older, adolescents and adults;
- 3 ml (corresponds to 300 mg) with a division price of 0.1 ml (corresponds to 10 mg) for children from 6 months to 4 years;
- 1 ml (corresponds to 100 mg) and divided into 0.05 ml (corresponds to 5 mg) for children from 1 to 6 months.
A measured dose of the drug is diluted in a glass of water or a baby bottle.
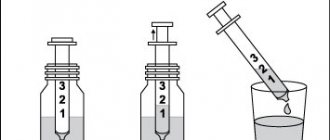
Instructions for dosing the solution using a measuring syringe
1. Open the bottle: to do this, press the cap and turn it counterclockwise.
2. Insert the syringe adapter into the neck of the bottle, make sure it is well fixed, then take the syringe and place it in the adapter.
3. Turn the bottle upside down.
4. Fill the syringe with a small amount of solution by pulling the plunger down, then push the plunger up (to remove air bubbles).
5. By pulling the plunger, fill the syringe with the solution until the division corresponds to the number of ml of the solution prescribed by the doctor.
6. Turn the bottle upside down and remove the syringe from the adapter.
7. Introduce the contents of the syringe into a glass of water or a baby bottle, pressing the plunger all the way.
8. Drink the entire contents of the glass (or baby bottle).
9. Rinse the syringe with water.
10. Close the bottle with a plastic cap.
Monotherapy
Adults and teenagers over 16 years of age.
The initial daily dose is 500 mg in 2 divided doses (250 mg 2 times a day). After 2 weeks, the dose can be increased to the initial therapeutic dose of 1000 mg (500 mg 2 times a day). The maximum daily dose is 3000 mg (1500 mg 2 times a day).
As part of complex therapy
Children from 1 to 6 months.
The initial therapeutic dose is 7 mg/kg 2 times a day. Depending on clinical effectiveness and tolerability, the dose may be increased to 21 mg/kg 2 times a day. Dose changes should not exceed ±7 mg/kg 2 times a day every 2 weeks. The minimum effective dose should be prescribed.
Recommended dosages for children from 1 to 6 months
| Body weight, kg | Initial dose: 7 mg/kg 2 times a day | Maximum dose - 21 mg/kg 2 times a day |
| 4 | 28 mg (0.3 ml) 2 times a day | 84 mg (0.85 ml) 2 times a day |
| 5 | 35 mg (0.35 ml) 2 times a day | 105 mg (1.05 ml) 2 times a day |
| 7 | 49 mg (0.5 ml) 2 times a day | 147 mg (1.5 ml) 2 times a day |
Children from 6 months to 11 years and adolescents from 12 to 17 years weighing less than 50 kg.
Treatment should begin with a dose of 20 mg/kg, divided into 2 doses (10 mg/kg 2 times a day). Depending on the clinical response and tolerability of the drug, the daily dose can be increased to 30 mg/kg 2 times a day. The dose can be changed by 20 mg/kg (10 mg/kg 2 times a day) of body weight every 2 weeks. The minimum effective dose should be used.
Recommended dosages for children (from 6 months) and adolescents (up to 17 years)
| Body weight, kg | Initial dose: 10 mg/kg 2 times a day | Maximum dose - 30 mg/kg 2 times a day |
| 6* | 60 mg (0.6 ml) 2 times a day | 180 mg (1.8 ml) 2 times a day |
| 10* | 100 mg (1 ml) 2 times a day | 300 mg (3 ml) 2 times a day |
| 15* | 150 mg (1.5 ml) 2 times a day | 450 mg (4.5 ml) 2 times a day |
| 20* | 200 mg (2 ml) 2 times a day | 600 mg (6 ml) 2 times a day |
| 25 | 250 mg 2 times a day | 750 mg 2 times a day |
| from 50** | 500 mg 2 times a day | 1500 mg 2 times a day |
* For children weighing 25 kg or less, it is preferable to start treatment with Keppra®, oral solution, 100 mg/ml.
** The dosage for children and adolescents weighing more than 50 kg is the same as for adults.
Due to the lack of the required dosage, the tablets are not intended for the treatment of children weighing less than 25 kg, when a dose is prescribed less than 250 mg, as well as for patients who have difficulty swallowing them. In these cases, it is recommended to begin treatment by taking the drug in the form of an oral solution.
Adults and adolescents (from 12 to 17 years old) weighing more than 50 kg.
The initial daily dose is 1000 mg in 2 divided doses (500 mg 2 times a day). Depending on the clinical response and tolerability of the drug, the daily dose can be increased to a maximum of 3000 mg (1500 mg 2 times a day). The dose can be changed to 500 mg 2 times a day every 2–4 weeks.
Special patient groups
Renal dysfunction.
Since levetiracetam is excreted from the body by the kidneys, when prescribing the drug to patients with renal failure, the dose should be adjusted depending on the creatinine clearance.
Creatinine clearance for men can be calculated from serum creatinine concentration using the following formula:
Cl creatinine, ml/min=(140−age, years)×body weight, kg/72×Cl serum creatinine, mg/dl
Creatinine clearance for women can be calculated by multiplying the resulting value by a factor of 0.85.
Creatinine clearance is then adjusted for body surface area (BSA) using the following formula:
Cl creatinine, ml/min/1.73 m2=Cl creatinine, ml/min×1.73/BSA of object, m2
Dose adjustment for adults
| Kidney failure | Cl creatinine, ml/min/1.73 m2 | Dosage regimen |
| Absent (normal) | >80 | from 500 to 1500 mg 2 times a day |
| Lightweight | 50–79 | from 500 to 1000 mg 2 times a day |
| Moderate | 30–49 | from 250 to 750 mg 2 times a day |
| Heavy | <30 | from 250 to 500 mg 2 times a day |
| End stage (hemodialysis patients) | — | from 500 to 1000 mg 1 time per day* |
* On the first day of treatment with levetiracetam, a loading dose of 750 mg is recommended. After dialysis, an additional dose of 250–500 mg is recommended.
In children with renal failure, dose adjustment of levetiracetam should be made taking into account the degree of renal failure.
Creatinine clearance (ml/min/1.73 m2) can be estimated from serum creatinine (mg/dL) for adolescents, children and neonates using the following formula (Schwartz formula):
Cl creatinine, ml/min/1.73 m2=height, cm×ks/Cl serum creatinine, mg/dl
ks=0.45 for children under 1 year; ks=0.55 for children under 13 years of age and female adolescents; ks=0.7 for male adolescents.
Dose adjustment for newborns, children and adolescents weighing less than 50 kg
| Kidney failure | Cl creatinine, ml/min/1.73 m2 | Dosage regimen | |
| from 1 to 6 months | from 6 months | ||
| Absent (normal) | >80 | 7–21 mg/kg (0.07–0.21 ml/kg) 2 times a day | 10–30 mg/kg (0.1–0.3 ml/kg) 2 times a day |
| Lightweight | 50–79 | 7–14 mg/kg (0.07–0.14 ml/kg) 2 times a day | 10–20 mg/kg (0.1–0.2 ml/kg) once a day |
| Moderate | 30–49 | 3.5–10.5 mg/kg (0.035–0.105 ml/kg) 2 times a day | 5–15 mg/kg (0.05–0.15 ml/kg) 2 times a day |
| Heavy | <30 | 3.5–7 mg/kg (0.035–0.07 ml/kg) 2 times a day | 5–10 mg/kg (0.1–0.2 ml/kg) 2 times a day |
| End stage (hemodialysis patients) | — | 7–14 mg/kg (0.07–0.14 ml/kg) 1 time per day* | 10–20 mg/kg (0.1–0.2 ml/kg) once a day** |
* On the first day of treatment with levetiracetam, a loading dose of 10.5 mg/kg (0.105 ml/kg) is recommended. After dialysis, an additional dose of 3.5–7 mg/kg (0.035–0.07 ml/kg) is recommended.
**On the first day of treatment with levetiracetam, a loading dose of 15 mg/kg (0.15 ml/kg) is recommended. After dialysis, an additional dose of 5–10 mg/kg (0.05–0.1 ml/kg) is recommended.
Liver dysfunction.
Patients with mild to moderate liver dysfunction do not require dosage adjustment. In patients with decompensated liver function and renal failure, the decrease in creatinine clearance may not fully reflect the severity of renal failure. In such cases, with creatinine clearance <60 ml/min/1.73 m2, it is recommended to reduce the daily dose by 50%.
Keppra®
Pharmacodynamics
Levetiracetam, the active substance of Keppra®, is a pyrrolidium derivative (S-enantiomer of α-ethyl-2-oxo-1-pyrrolidine acetamide) and differs in chemical structure from known antiepileptic drugs.
Mechanism of action
The mechanism of action of levetiracetam is not fully understood, but it is obvious that it differs from the mechanism of action of known antiepileptic drugs. In vitro and in vivo experiments have shown that levetiracetam does not affect basic cell characteristics and normal transmission.
In vitro studies have shown that levetiracetam affects the intraneuronal concentration of Ca2+ ions, partially inhibiting Ca2+ current through N-type channels and reducing the release of calcium from intraneuronal stores. In addition, levetiracetam partially restores currents through GABA- and glycine-dependent channels reduced by zinc and β-carbolines.
One of the proposed mechanisms is based on proven binding to the synaptic vesicle glycoprotein SV2A, contained in the gray matter of the brain and spinal cord. It is believed that in this way an anticonvulsant effect is realized, which is expressed in counteracting the hypersynchronization of neural activity.
Levetiracetam also acts on GABA receptors and glycine receptors, modulating these receptors through various endogenous agents. Does not alter normal neurotransmission, but suppresses epileptiform neuronal bursts induced by the GABA agonist bicuculline and excitation of glutamate receptors.
Pharmacodynamic effects
The activity of the drug has been confirmed against both focal and generalized epileptic seizures (epileptiform manifestations/photoparokeismal reaction). Levetiracetam induces protection against seizures in a variety of animal models.
Adjunctive therapy for partial seizures with or without secondary generalization in adults, adolescents and children over 1 month of age with epilepsy:
In adults, the effectiveness of levetiracetam was shown in 3 double-blind, placebo-controlled studies. It was shown that the proportion of patients who demonstrated a 50% or greater reduction in the frequency of partial seizures per week from baseline when taking continuous levetiracetam doses of 1000 mg, 2000 mg or 3000 mg in two doses for 12-14 weeks was 27.7%. , 31.6% and 41.3%, respectively, and 12.6% in patients taking placebo.
Pediatric population
The effectiveness of levetiracetam in patients aged 4 to 16 years was established in a double-blind, placebo-controlled study of 14 weeks duration, including 198 patients. The dose of levetiracetam was 60 mg/kg/day in two divided doses.
44.6% of patients taking levetiracetam and 19.6% of patients taking placebo showed a 50% or greater reduction in the frequency of partial seizures per week from baseline.
During the treatment period, 11.4% of patients were seizure-free for at least 6 months and 7.2% were seizure-free for at least one year.
The effectiveness of levetiracetam in patients aged one month to 4 years was established in a double-blind, placebo-controlled study of 116 patients with a treatment duration of 5 days. The dose of levetiracetam as an oral solution for infants from one to 6 months was 20 mg/kg/day in two divided doses, followed by titration to 40 mg/kg/day, for infants and children from 6 months to 4 years - 25 mg/day. kg/day in two doses, followed by titration to 50 mg/kg/day.
During the initial efficacy assessment, the response rate (percentage of patients with a 50% or greater reduction in partial seizure frequency per day from baseline) was determined using an anonymous reader during 48-hour video electroencephalography. The efficacy rate is based on an analysis of 109 patients who underwent electroencephalography for at least 24 hours. Responders were 43.6% of patients taking levetiracetam and 19.6% of patients taking placebo. Over the course of long-term treatment, 8.6% of patients were seizure-free for at least 6 months and 7.8% for at least 1 year.
Monotherapy for partial seizures with or without secondary generalization in patients over 16 years of age with newly diagnosed epilepsy
The efficacy of levetiracetam as monotherapy was comparable to that of controlled-release carbamazepine in a parallel group, double-blind study of 576 patients over 16 years of age with newly or recently diagnosed epilepsy with unprovoked partial seizures or generalized tonic-clonic seizures. Patients were randomly selected to receive controlled-release carbamazepine 400–200 mg/day or levetiracetam 1000–3000 mg/day. Duration of treatment was up to 121 weeks depending on response.
Seizure freedom at 6 months was observed in 73% of patients taking levetiracetam and 72.8% of patients taking controlled-release carbamazepine. The agreed absolute difference between treatments was 0.2% (95% confidence interval -7.8 to 8.2). More than half of the patients were seizure-free at 12 months (56.6% of patients on levetiracetam and 58.5% on controlled-release carbamazepine, respectively).
When the study was conducted in clinical practice, concomitant antiepileptic drugs could be discontinued in a limited number of patients who responded to adjunctive levetiracetam therapy (36 of 69 adult patients).
Additional therapy of myoclonic seizures in adults and adolescents from 12 years of age with juvenile myoclonic epilepsy
The effectiveness of levetiracetam was established in a double-blind, placebo-controlled study lasting 16 weeks in patients over 12 years of age with idiopathic generalized epilepsy with various myoclonic seizure syndromes. Most patients had juvenile myoclomic epilepsy. The dose of levetiracetam was 3000 mg/day in two divided doses. 58.3% of patients taking levetiracetam and 23.3% of patients taking placebo had at least a 50% reduction in myoclonic seizures over a week. During continuous long-term treatment, 28.6% of patients were free of myoclonic seizures for at least 6 months and 21% of patients for at least one year.
Additional therapy for primary generalized convulsive (tonic-clonic) seizures in adults and adolescents over 12 years of age with idiopathic generalized epilepsy
The effectiveness of levetiracitam was established during a 24-week, double-blind, placebo-controlled study that included adults, adolescents and a limited number of children with idiopathic generalized epilepsy with primary generalized tonic-clonic seizures, with various syndromes (juvenile myoclonic epilepsy, juvenile absence epilepsy, childhood absence epilepsy or epilepsy with generalized tonic-clonic seizures on awakening).
In this study, the daily dose of levetiracetam was 3000 mg/day for adults and adolescents or 60 mg/kg/day for children in two divided doses. 72.2% of patients taking levetiracetam and 45.2% of patients taking placebo showed a 50% or greater reduction in seizure frequency within a week in patients with primary generalized tonic-clonic seizures.
During continuous long-term treatment, 47.4% of patients were free of tonic-clonic seizures for at least 6 months, and 31.5% of patients were free of tonic-clonic seizures for at least one year.
Keppra solution for internal use 100 mg/ml fl 300 ml
Contraindications
- children under 1 month of age (for solution) (the safety and effectiveness of the drug have not been established);
impaired tolerance to fructose (for solution);
The drug should be prescribed with caution to elderly patients (over 65 years old), with liver diseases in the stage of decompensation, or renal failure.
Directions for use and doses
The daily dose is divided into 2 equal doses.
Monotherapy
For adults and adolescents over 16 years of age, the drug is prescribed in the form of tablets or oral solution at an initial dose of 500 mg, divided into 2 doses (250 mg 2 times a day). After 2 weeks, the dose can be increased to the initial therapeutic dose of 1 g (500 mg 2 times a day). The maximum daily dose is 3 g (1.5 g 2 times a day).
As part of complex therapy
For children aged 1 month to 6 months, the drug is prescribed in the form of an oral solution. The initial therapeutic dose is 7 mg/kg 2 times/day. Depending on clinical effectiveness and tolerability, the dose may be increased to 21 mg/kg 2 times/day. Dose changes should not exceed plus or minus 7 mg/kg 2 times/day every 2 weeks. The minimum effective dose should be prescribed.
Recommendations for dosing of Keppra® in the form of oral solution for children under 6 months of age are presented in the table.
| Body mass | Initial dose: 7 mg/kg 2 times/day | Maximum dose: 21 mg/kg 2 times/day |
| 4 kg | 28 mg (0.3 ml) 2 times/day | 84 mg (0.85 ml) 2 times/day |
| 5 kg | 35 mg (0.35 ml) 2 times/day | 105 mg (1.05 ml) 2 times/day |
| 7 kg | 49 mg (0.5 ml) 2 times/day | 147 mg (1.5 ml) 2 times/day |
In children aged 6 months to 23 months, children aged 2 years to 11 years and adolescents from 12 years to 17 years weighing less than 50 kg, treatment should begin with a dose of 10 mg/kg body weight, divided into 2 doses (10 mg/kg body weight 2 times/day).
Depending on the clinical response and tolerability of the drug, the daily dose can be increased to 30 mg/kg 2 times a day. The dose can be changed by 10 mg/kg body weight every 2 weeks. The minimum effective dose should be used. The dose for children weighing 50 kg or more is the same as for adults.
Recommended doses for children aged 6 months and older and adolescents are presented in the table.
| Body mass | Initial dose: 10 mg/kg 2 times/day | Maximum dose: 30 mg/kg 2 times/day |
| 6 kg | 60 mg (0.6 ml) 2 times/day | 180 mg (1.8 ml) 2 times/day |
| 10 kg | 100 mg (1 ml) 2 times/day | 300 mg (3 ml) 2 times/day |
| 15 kg | 150 mg (1.5 ml) 2 times/day | 450 mg (4.5 ml) 2 times/day |
| 20 kg | 200 mg (2 ml) 2 times/day | 600 mg (6 ml) 2 times/day |
| 25 kg | 250 mg 2 times/day | 750 mg 2 times/day |
| from 50 kg | 500 mg 2 times/day | 1500 mg 2 times/day |
In children over 4 years of age, treatment should begin with a daily dose of 20 mg/kg body weight, divided into 2 doses (10 mg/kg body weight 2 times a day).
The dose can be changed by 20 mg/kg body weight every 2 weeks until the recommended daily dose is reached - 60 mg/kg body weight (30 mg/kg body weight 2 times a day). If the recommended daily dose is not tolerated, it may be reduced. The minimum effective dose should be used. The drug should be prescribed in the most appropriate dosage form and dose depending on the patient’s body weight and the required therapeutic dose.
Children weighing ≤ 20 kg are recommended to begin treatment with the drug in the form of an oral solution.
For children weighing > 50 kg, dosage is carried out according to the scheme given for adults.
For adults and adolescents over 16 years of age weighing more than 50 kg, treatment should begin with a daily dose of 1 g, divided into 2 doses (500 mg 2 times a day). Depending on the clinical response and tolerability of the drug, the daily dose can be increased to a maximum of 3 g (1.5 g 2 times a day). The dose can be changed to 500 mg 2 times a day every 2-4 weeks.
Since levetiracetam is excreted by the kidneys, when the drug is prescribed to elderly patients and patients with renal failure, the dose should be adjusted depending on the value of CC.
CC can be calculated based on serum creatinine concentration using the following formula.
For men:
CC (ml/min)= [140-age (years)] × body weight (kg)/72 × serum creatinine (mg/dl)
For women: obtained value x 0.85
| Kidney failure | CC (ml/min) | Dose and frequency of administration |
| Norm | >80 | 500-1500 mg 2 times/day |
| Latent | 50-79 | 500-1000 mg 2 times/day |
| Compensated | 30-49 | 250-750 mg 2 times/day |
| Intermittent | <30 | 250-500 mg 2 times/day |
| End stage (patients on hemodialysis)* | — | 500-1000 mg 1 time/day** |
* - on the first day of treatment with Keppra®, a loading dose of 750 mg is recommended.
** - after dialysis, it is recommended to take an additional dose of 250-500 mg.
In children with renal impairment, dosage adjustments of levetiracetam should be made based on the degree of renal impairment, using recommendations given for adults.
Patients with mild to moderate liver dysfunction do not require dosage adjustment. In patients with decompensated liver dysfunction and renal failure, the CC value may not reflect the true degree of renal dysfunction, therefore, when CC <70 ml/min, a reduction in the daily dose by 50% is recommended.
Rules for using the drug
Dosing of the solution is carried out using a measuring syringe with a nominal capacity of 10 ml (corresponding to 1 g of levetiracetam) and with a division value of 25 mg (corresponding to 0.25 ml), included in the delivery package of the drug. The measured dose of the drug is diluted in a glass of water (200 ml).
To dispense the solution using a measuring syringe, open the bottle: to do this, press the cap and turn it counterclockwise. Insert the syringe adapter into the neck of the bottle, then take the syringe and place it in the adapter. Turn the bottle upside down. Fill the syringe with a small amount of solution by pulling the plunger down, then push the plunger up to remove any air bubbles. By pulling the plunger, fill the syringe with the solution until the division corresponds to the number of ml of solution prescribed by the doctor. Remove the syringe from the adapter. Introduce the contents of the syringe into a glass of water, pressing the plunger all the way. You should drink the entire contents of the glass. Then rinse the syringe with water and close the bottle with a plastic cap.
Storage conditions
At a temperature not exceeding 30 °C, in a place protected from light.
Keep out of the reach of children.
Best before date
3 years. Do not use after the expiration date.
Use an opened bottle within seven months.
special instructions
If you need to stop taking the drug, it is recommended to discontinue it gradually, reducing the single dose by 500 mg every 2-4 weeks. In children, the dose reduction should not exceed 10 mg/kg body weight 2 times a day every 2 weeks.
It is advisable to gradually discontinue concomitant antiepileptic drugs (during the period of transfer of patients to levetiracetam).
For patients with kidney disease and decompensated liver disease, a kidney function test is recommended before starting treatment. If renal function is impaired, dose adjustment may be required.
Due to reports of suicide, suicidal ideation and suicide attempts during treatment with levetiracetam, patients should be warned to immediately notify their physician of any symptoms of depression or suicidal ideation.
The oral solution contains maltitol, therefore, patients with fructose intolerance should not take Keppra® in the appropriate dosage form.
Use in pediatrics
Available information on the use of the drug in children does not indicate any negative effect on development and puberty. However, the long-term effects of treatment on children's learning ability, intellectual development, growth, endocrine gland function, sexual development and fertility remain unknown.
Description
Anticonvulsant drug.
Dosage form
The solution for oral administration is clear, almost colorless, with a characteristic odor.
Use in children
Contraindicated: children under 1 month of age (the safety and effectiveness of the drug have not been established).
Action
An antiepileptic drug, a derivative of pyrrolidone (S-enantiomer of α-ethyl-2-oxo-1-pyrrolidine acetamide), differs in chemical structure from known antiepileptic drugs. The mechanism of action of levetiracetam is not fully understood, but it is obvious that it differs from the mechanism of action of known antiepileptic drugs.
In vitro studies have shown that levetiracetam affects the intraneuronal concentration of Ca2+ ions, partially inhibiting Ca2+ current through N-type channels and reducing the release of calcium from intraneuronal stores. In addition, levetiracetam partially restores currents through GABA- and glycine-dependent channels reduced by zinc and β-carbolines.
One of the proposed mechanisms is based on proven binding to the synaptic vesicle glycoprotein SV2A, contained in the gray matter of the brain and spinal cord. It is believed that in this way an anticonvulsant effect is realized, which is expressed in counteracting the hypersynchronization of neural activity. Does not alter normal neurotransmission, but suppresses epileptiform neuronal bursts induced by the GABA agonist bicuculline and excitation of glutamate receptors. The activity of the drug has been confirmed against both focal and generalized epileptic seizures (epileptiform manifestations/photoparoxysmal reaction).
Side effects
Possible side effects are listed below by body system and frequency of occurrence: very often (>1/10), often (>1/100, <1/10).
From the side of the central nervous system: very often - drowsiness, asthenic syndrome; often - amnesia, ataxia, convulsions, dizziness, headache, hyperkinesia, tremor, imbalance, decreased concentration, memory impairment, agitation, depression, emotional lability, mood swings, hostility/aggressiveness, insomnia, nervousness, irritability, personality disorders, thinking disorder; in some cases - paresthesia, behavioral disorders, anxiety, restlessness, confusion, hallucinations, irritability, psychotic disorders, suicide, suicide attempts and suicidal intentions.
From the side of the organ of vision: often - diplopia, impaired accommodation.
From the respiratory system: often - increased cough.
From the digestive system: often - abdominal pain, diarrhea, dyspepsia, nausea, vomiting, anorexia, weight gain; in some cases - pancreatitis, liver failure, hepatitis, changes in liver function tests, weight loss.
Dermatological reactions: often - skin rash, eczema, itching; in some cases - alopecia (in some cases, hair restoration was observed after discontinuation of the drug), Stevens-Johnson syndrome, erythema multiforme, toxic epidermal necrolysis.
From the hematopoietic system: in some cases - leukopenia, neutropenia, pancytopenia (in some cases with suppression of bone marrow function), thrombocytopenia.
Other: in some cases - infections, nasopharyngitis, myalgia.
Use during pregnancy and breastfeeding
Adequate and strictly controlled clinical studies on the safety of levetiracetam in pregnant women have not been conducted, so the drug should not be prescribed during pregnancy unless absolutely necessary.
Physiological changes in a woman's body during pregnancy can affect plasma concentrations of levetiracetam, as well as other antiepileptic drugs. During pregnancy, a decrease in plasma concentrations of levetiracetam was observed. This decrease is more pronounced in the first trimester (up to 60% of the baseline concentration in the period preceding pregnancy).
Treatment with levetiracetam in pregnant women should be carried out under special supervision. It should be borne in mind that interruptions in antiepileptic therapy can lead to a worsening of the disease, which is harmful for both the mother and the fetus.
Levetiracetam is excreted in breast milk, so breastfeeding during treatment with the drug is not recommended. However, if treatment with levetiracetam is necessary during lactation, the risk/benefit ratio of treatment should be carefully weighed against the importance of breastfeeding.
Interaction
- Levetiracetam does not interact with anticonvulsants (phenytoin, carbamazepine, valproic acid, phenobarbital, lamotrigine, gabapentin and primidone).
- Levetiracetam at a daily dose of 1 g does not change the pharmacokinetics of oral contraceptives (ethinyl estradiol and levonorgestrel).
- Levetiracetam at a daily dose of 2 g does not change the pharmacokinetics of warfarin and digoxin.
- Digoxin, oral contraceptives and warfarin do not affect the pharmacokinetics of levetiracetam.
- When taken together with topiramate, the likelihood of developing anorexia is higher.
- The completeness of absorption of levetiracetam does not change under the influence of food, but the rate of absorption is slightly reduced.
- There are no data on the interaction of levetiracetam with alcohol.
Overdose
Symptoms: drowsiness, anxiety, aggressiveness, depression of consciousness, respiratory depression, coma.
Treatment: in the acute period - artificial induction of vomiting and gastric lavage, followed by the administration of activated charcoal. There is no specific antidote for levetiracetam. If necessary, symptomatic treatment is carried out in a hospital setting using hemodialysis (dialysis efficiency for levetiracetam is 60%, for its primary metabolite - 74%).
Impact on the ability to drive vehicles and operate machinery
The effect of Keppra® on the ability to drive vehicles and operate machinery has not been specifically studied. However, due to varying individual sensitivity to the drug on the part of the central nervous system, during the treatment period it is necessary to refrain from driving vehicles and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.