Nosological classification (ICD-10)
- A36.9 Diphtheria, unspecified
- A37 Whooping cough
- A39.0 Meningococcal meningitis
- A64 Sexually transmitted diseases, unspecified
- A74.9 Chlamydial infection, unspecified
- B58 Toxoplasmosis
- H66 Suppurative and unspecified otitis media
- J01 Acute sinusitis
- J03.9 Acute tonsillitis, unspecified (angina agranulocytic)
- J15.6 Pneumonia caused by other aerobic gram-negative bacteria
- J15.7 Pneumonia caused by Mycoplasma pneumoniae
- J16.0 Pneumonia caused by chlamydia
- J32 Chronic sinusitis
- J35.0 Chronic tonsillitis
- J42 Chronic bronchitis, unspecified
- K12.2 Cellulitis and abscess of the oral cavity
- L02 Skin abscess, boil and carbuncle
- L08.9 Local infection of skin and subcutaneous tissue, unspecified
- M13.9 Arthritis, unspecified
- M79.0 Rheumatism, unspecified
- M86 Osteomyelitis
- N34 Urethritis and urethral syndrome
- N39.0 Urinary tract infection without established location
- N41.0 Acute prostatitis
- N41.1 Chronic prostatitis
Composition and release form
| Film-coated tablets | 1 table |
| spiramycin | 3 million IU |
| excipients: MCC; polyplasdone ICS EL-10 (crospovidone); sodium carboxymethyl starch (Primogel); PVP (povidone); aerosil (colloidal silicon dioxide); magnesium stearate; Opadry II |
in a blister pack 5 pcs.; in a cardboard pack 2 packs or in a blister pack 10 pcs.; in a cardboard pack 1 package or in dark glass jars of 10 pcs.; in a cardboard pack 1 jar.
Use during pregnancy
Spiramycin-Vero is used during pregnancy according to indications. No teratogenic effect of the drug has been detected. Its use is contraindicated during lactation, since it can be excreted in milk. During this period, breastfeeding stops.
For hospital-acquired pneumonia in pregnant women, the drugs of choice are the latest generation cephalosporins clavulanate , and reserve antibiotics: Spiramycin , Clindamycin , Vancomycin .
The prescription regimens for Spiramycin for this disease are different. It is used in the form of tablets of 3 million ME 3 times a day, in the form of infusions of 3 million ME 3 times a day intravenously. It may be prescribed in the first 2-4 days in the form of infusions, and then orally for 7-10 days. Moreover, parenteral (IV) antibiotics for the treatment of pneumonia have no advantages over oral ones.
For pyelonephritis in pregnant women, the drug is prescribed at a dose of 1.5-3 million IU 3 times a day. For ureaplasma infection in pregnant women, this drug is also prescribed, however, information has appeared on the identification of mycoplasmas and ureplasmas resistant to spiramycin.
Treatment of pregnant women with chlamydia - 3 million IU orally 3 times a day for 10 days. In the treatment of this infection, spiramycin has greater therapeutic efficacy than erythromycin .
Pharmacodynamics
An antibiotic from the macrolide group, it acts bacteriostatically (when used in high doses it can act bactericidal against more sensitive strains): it suppresses protein synthesis in the microbial cell due to reversible binding to the 50S ribosomal subunit, which leads to blockade of transpeptidation and translocation reactions. Unlike 14-membered macrolides, it is able to bind not to one, but to three (I–III) domains of the subunit, which possibly provides more stable binding to the ribosome and, consequently, a longer antibacterial effect. Can accumulate in high concentrations in the bacterial cell.
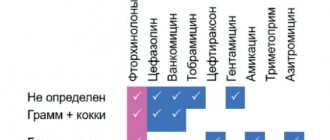
The following microorganisms are usually sensitive to the drug: Staphylococcus spp. (including strains of Staphylococcus aureus sensitive to methicillin), Streptococcus spp., Neisseria meningitidis, Neisseria gonorrhoeae, Bordetella pertussis, Corynebacterium diphtheriae, Listeria monocytogenes, Clostridium spp., Mycoplasma pneumoniae, Chlamydia spp., Legionella pneumophila, Treponema spp. ., Leptospira spp., Campylobacter spp., Toxoplasma gondii. Moderately sensitive: Haemophilus influenzae. Resistant to spiramycin: Enterobacteriaceae spp., Pseudomonas spp..
There is cross-resistance between spiramycin and erythromycin.
S.V. Yakovlev MMA named after. THEM. Sechenov, Moscow
Macrolide antibiotics have been used in clinical practice for about 50 years. The first drug of this class, erythromycin, was introduced into practice in 1952, three years later another drug, spiramycin, appeared. Until the mid-1980s. macrolides have been used to a limited extent, mainly for upper respiratory tract infections. However, in recent years, interest in these drugs has expanded significantly, primarily as a result of changes in the structure of infectious diseases and an increase in the frequency of infections caused by atypical microorganisms with predominantly intracellular localization - chlamydia and mycoplasma. In addition, the activity of macrolide antibiotics against Legionella, Helicobacter pylori, and some opportunistic pathogens in patients with AIDS was revealed. Simultaneously with the introduction into practice of new semi-synthetic macrolides with improved pharmacokinetic properties (azithromycin, clarithromycin, roxithromycin), a revaluation of the “early” macrolides, and primarily spiramycin, occurred. In terms of antimicrobial properties, pharmacokinetics and pharmacodynamics parameters, this drug turned out to be similar to new semisynthetic drugs; in clinical effectiveness and tolerability it was not inferior to them, and in a number of parameters it was superior. Due to these qualities, spiramycin is currently one of the most frequently prescribed drugs among macrolide antibiotics for community-acquired respiratory infections in adults and children. The most complete information about spiramycin can be found in the monograph [1] and a number of systematic reviews [2-6]. The main advantage of macrolides, which allows them to maintain a leading position in the treatment of respiratory infections, is their good tolerability and high effectiveness in acute uncomplicated infections, comparable to the effectiveness of b-lactam antibiotics. At the same time, unlike b-lactams, macrolides penetrate into the cells of the macroorganism, as a result of which they can affect intracellular bacteria, primarily Chlamydia pneumoniae. The modern classification of macrolides involves dividing drugs depending on their chemical structure (Table 1) and origin (natural or semi-synthetic). Depending on the number of carbon atoms in the macrocyclic lactone ring, 14-, 15- and 16-membered macrolides are distinguished. Semi-synthetic macrolides (azithromycin, clarithromycin, roxithromycin) differ from natural ones by higher activity against Haemophilus influenzae and gram-positive cocci (however, with respect to the latter, the differences are minimal), as well as prolonged pharmacokinetics. At the same time, natural 16-membered macrolides can retain activity against pneumococci and pyogenic streptococci that are resistant to erythromycin and semisynthetic macrolides. Spiramycin is a natural 16-membered macrolide and is characterized by natural antimicrobial activity similar to erythromycin and other macrolides. The antibiotic is active against most gram-positive aerobic and anaerobic microorganisms and some gram-negative bacteria and protozoa (Table 2). Gram-negative bacteria of the family Enterobacteriaceae and non-fermenting microorganisms are resistant to spiramycin. Among the causative agents of respiratory infections, strains resistant to spiramycin can be identified, but their number is small. Currently in Russia, the level of resistance of pneumococci and b-hemolytic streptococci to spiramycin does not exceed 10% and is usually lower than to erythromycin. If among 14- and 15-membered macrolides the resistance of pneumococci and streptococci is cross-resistance (strains resistant to erythromycin are always also resistant to clarithromycin and azithromycin), then some erythromycin-resistant bacteria remain sensitive to spiramycin and other 16-membered macrolides. The resistance of atypical microorganisms (chlamydia, mycoplasma) to spiramycin has not yet been described. In terms of the level of natural activity, spiramycin is slightly inferior to erythromycin and other 14- and 15-membered macrolides. However, studies conducted in vivo and clinical data indicate the high effectiveness of spiramycin, including for infections caused by poorly susceptible microorganisms (for example, H. influenzae, L. pneumophila). This discrepancy between in vitro and clinical data allowed us to make a statement about the “phenomenon” or “paradox” of spiramycin. The reasons for this phenomenon lie in the special biological and pharmacokinetic properties of the drug. Several factors can be identified that explain the higher clinical effectiveness of spiramycin: • high tissue and intracellular concentrations; • pronounced post-antibiotic effect; • immunomodulatory properties.
Tissue and intracellular concentrations Good penetration of spiramycin into various tissues was noted, with tissue concentrations being 5-10 times higher than serum concentrations and in most cases higher than MIC90 values even for weakly sensitive microorganisms (Table 3). When using spiramycin, high intracellular concentrations are created, while the concentrations of the drug in alveolar macrophages and polymorphonuclear neutrophils are 10-20 times higher than extracellular concentrations. Accumulating in circulating and tissue macrophages, spiramycin penetrates with them into the site of infection, where high bactericidal concentrations of the drug are created. Spiramycin is active in cells. Spiramycin concentrations in tissues are maintained at therapeutic levels for a long time as a result of slow release from cells. Effective intracellular and tissue concentrations of spiramycin persist several times longer than erythromycin [7]. The postantibiotic effect (PAE) is defined as the continued inhibition of bacterial growth in vitro when the antibiotic is removed from the incubation medium. The clinical significance of PAE is not clearly established, but it may be important in explaining the fact that spiramycin may be given at longer dosing intervals (12 hours) than those calculated on the basis of half-life. Spiramycin is characterized by the longest PAE among macrolides against S.pneumoniae and S.aureus (4-9 and 9-12 hours, respectively) [4, 8, 9]. Another important clinical significance of PAE is the induced decrease in microbial virulence during this period as a result of adhesion disturbances, decreased tissue invasion, and changes in bacterial sensitivity to phagocytosis. As a result, during the period of PAE, microorganisms are more susceptible to the bactericidal action of neutrophils.
Immunomodulatory properties The effect of antibacterial drugs on specific and nonspecific defense reactions of the macroorganism is an important component of anti-infective resistance. Spiramycin increases the activity of T-killers, accumulates in neutrophils and macrophages, enhances their phagocytic activity and migration to the site of inflammation. In addition, the drug affects oxidative reactions in phagocytes and promotes their degranulation, increases the production of anti-inflammatory cytokine (interleukin-10) by monocytes, reduces the production of pro-inflammatory cytokines by monocytes (interleukin-1, TNF) and lymphocytes (interleukin-2), reduces the formation of inflammatory mediators – prostaglandins, leukotrienes and thromboxanes [4, 10]. The anti-inflammatory effect occurs even at subtherapeutic concentrations and is comparable to the effect of non-steroidal anti-inflammatory drugs [11]. Thus, the listed properties explain the high bactericidal activity and high clinical effectiveness of spiramycin against most microorganisms, even strains that are weakly sensitive in vitro [2, 5]. An additional advantage of spiramycin, which explains the continued interest in the drug on the part of clinicians, is the low frequency of resistant strains of respiratory pathogens (S.pneumoniae, S.pyogenes, S.aureus, H.influenzae) despite 50 years of use in medicine.
Clinical use Spiramycin is widely used in clinical practice (Table 4). The main area of application of spiramycin is community-acquired infections of the upper and lower respiratory tract in adults and children. Over 50 years of use in medicine, extensive clinical experience has been accumulated in its use in various categories of patients, including children, pregnant women, and the elderly. The clinical effectiveness of spiramycin has been studied in numerous clinical studies. In acute streptococcal tonsillopharyngitis, the same effectiveness of spiramycin is shown in comparison with phenoxymethylpenicillin and erythromycin, in acute rhinosinusitis - in comparison with doxycycline. In non-severe community-acquired pneumonia, the effectiveness of spiramycin was comparable to amoxicillin and cefuroxime axetil and superior to erythromycin [1, 5]. The high clinical effectiveness of spiramycin has been established in severe community-acquired pneumonia, including that caused by Legionella [12]. The high clinical effectiveness of spiramycin has been shown in controlled studies in non-gonococcal urethritis and urogenital chlamydia. In chlamydial cervicitis, the effectiveness of spiramycin was the same as doxycycline [13]. The domestic literature provides data on the treatment of 40 patients with acute chlamydial urethritis with spiramycin (3 million IU every 8 hours for 10 days), while the clinical effectiveness was 100%, and eradication of the pathogen was observed in 27 out of 30 patients [14]. Another study [15] studied the effectiveness of spiramycin in 30 patients with reactive urogenic arthritis of chlamydial etiology. During the treatment, a positive clinical effect was observed in 93.3%, eradication was confirmed in 66.7% of patients. Spiramycin has been shown to be highly effective in the treatment of dental infections. Spiramycin, due to the creation of high concentrations in saliva and long-lasting therapeutic concentrations in the gums and bones, has been successfully used to treat periodontitis [16]. Spiramycin has been found to be more effective in treating periodontitis compared to tetracycline and erythromycin [17-18]. A number of studies have shown the high clinical effectiveness of the therapeutic and prophylactic combined use of spiramycin and metronidazole in dentistry [1]. In most clinical studies, spiramycin was well tolerated. Macrolide antibiotics are considered one of the safest among antibacterial agents, among them spiramycin and roxithromycin are the most well-tolerated drugs with a low incidence of adverse reactions. Spiramycin is better tolerated compared to erythromycin, clarithromycin and azithromycin, since, unlike them, it does not have a prokinetic effect and is less likely to cause gastrointestinal reactions such as diarrhea [19]. When using spiramycin, liver damage (cholestatic jaundice, hepatitis, increased transaminases) is also less likely (compared to erythromycin). Unlike 14-membered macrolides, spiramycin does not alter the metabolism of other drugs in the liver. There are virtually no drug interactions with its use, which is especially important in elderly patients taking other medications. Rovamycin is included in the list of additional drugs, and this point is a serious advantage when choosing an antibiotic (from those included in the list) for prescribing to this category of patients. The experiment showed that spiramycin has no teratogenic or embryotoxic properties. Controlled clinical studies have shown the fetal safety of long-term use of spiramycin in pregnant women [20]. Unlike other macrolides (erythromycin, clarithromycin, azithromycin), the safety of spiramycin for the fetus has been confirmed by many years of clinical experience in its use in pregnant women with toxoplasmosis. The recommended dosage regimen for spiramycin is 3 million IU (one tablet) every 12 hours, for parenteral use - 1.5 million IU (hourly infusion) every 8 hours. In children under 8 years of age, spiramycin is prescribed orally at the rate of 150 thousand IU/kg body weight per day (in two to three doses), in children over 8 years of age - 1.5 million IU every 12 hours. For toxoplasmosis in pregnant women, the daily dose of spiramycin is 9 million IU. To prevent meningococcal meningitis in persons in contact with the patient, spiramycin is administered orally for five days at a dose of 6 million IU per day. Spiramycin is prescribed regardless of food intake. Thus, 50 years of experience in the use of spiramycin in medicine allows us to state with high certainty that doctors have at their disposal a highly effective and safe macrolide antibiotic, the effectiveness of which does not decrease over time. In conclusion, we can once again emphasize the most important advantages of spiramycin, which distinguish it favorably from other macrolide antibiotics, such as: • special biological and pharmacokinetic properties (high and long-lasting tissue and intracellular concentrations, PAE, immunomodulatory activity), allowing to achieve a reliable clinical effect even against weakly sensitive pathogens; • maintaining activity against strains of pneumococci and streptococci resistant to erythromycin and other 14- and 15-membered macrolides - clarithromycin and azithromycin; • high clinical effectiveness in community-acquired pneumonia, not inferior to b-lactam antibiotics; • the highest effectiveness among macrolides for oral and periodontal infections; • better tolerability compared to erythromycin and other 14-membered (clarithromycin) and 15-membered (azithromycin) macrolides; • possibility of safe use in pregnant women; • the impossibility of creating generic forms of Rovamycin for technological reasons (today there is not a single generic drug in the world, which is the key to maintaining quality); • optimal exchange rate (17.22 euros) and cost of one day of treatment (2.46 euros) compared to other macrolide antibiotics registered in Russia.
Literature 1. Yakovlev S.V. Spiramycin. Monograph. M.: Rhone-Poulenc Rorer, 1997, 72 p. 2. Bergogne-Berezin E. Spiramycin concentrations in human respiratory tract: a review. J Antimicrob Chemother 1988;22(Suppl B):117-22. 3. Bergogne-Berezin E, Hamilton-Miller JMT. Overview of spiramycin in respiratory tract infections. Drug Invest 1993;6(Suppl 1):52-4. 4. Labro MT. Pharmacology of spiramycin: a comparison with other macrolides. Drug Invest 1993;6(Suppl 1):15-28. 5. Rubinstein E, Keller N. Spiramycin renaissance. J Antimicrob Chemother 1998;42:572-6. 6. Wise R. Clinical pharmacokinetics of spiramycin. Drug Invest 1993;6(Suppl 1):29-34. 7. Pocidalo J, Albert F, Desnottes J. Intraphagocytic penetration of macrolides: in-vivo camparison of erythromycin and spiramycin. J Antimicrob Chemother 1985;16 (Suppl A):167-73. 8. Chabbert YA. Early studies on in-vitro and experimental activity of spiramycin: e review. J Antimicrob Chemother 1988;22(SupplB):1-11. 9. Webster C, Chazanfar K, Slack R. Sub-inhibitory and post-antibiotic effects of spiramycin and erythromycin on Staphylococcus aureus. J Antimicrob Chemother 1988;22(Suppl B):33-9. 10. Prieto JP, Minguez F, Ortega P, et al. Camparative study of the effects of roxithromycin and other macrolides on polymorphonuclear cells in vitro. Brit J Clin Pract 1988;42 (Suppl):38-9. 11. Lukyanov S.V. Macrolides in the treatment of respiratory system infections. Clinical pharmacology of macrolides // Consilium Medicum. 2004. No. 6. P. 10. 12. Vachon F, Kernbaum S. Traitment des bronchopneumopathies aigues par la spiramycine IV. Infectiologie 1986;10:19-23. 13. Dylewski J, Clecner B, Dubois J, et al. Comparison of spiramycin and doxycycline for treatment of Chlamydia trachomatis genital infections. Antimicrob Agents Chemother 1993;37:1373-4. 14. Mashkilleyson A.L., Akovbyan V.A., Borisenko K.K. and others. Rovamycin in the treatment of urogenital chlamydia. III Russian National Congress “Man and Medicine”, April 16-20, 1996 M.: RC “Pharmmedinfo”, 1996, p. 164. 15. Chernykh T.M., Minakov E.V. The use of rovamycin in the treatment of reactive arthritis. III Russian National Congress “Man and Medicine”, April 16-20, 1996 M.: RC “Pharmmedinfo”, 1996, p. 234. 16. Modai J. The clinical use of macrolides. J Antimicrob Chemothe 1988;22(Suppl B):145-53. 17. Chin Quee T, Al-Joburi W, Lautar C, et al. Comparison od spiramycin and tetracycline in the treatment of advanced chronic periodontitis. J Antimicrob Chemother 1988;22(Suppl B):171-7. 18. Mills WH, Yhompson GW, Beagrie GS. Clinical evaluation of spiramycin and erythromycin in control of periodontal disease. J Clin Periodontol 1979;6:308-16. 19. Pilot MA, Qin XY. Macrolides and gastrointestinal motility. J Antimicrob Chemother 1988;22 (Suppl B):201-6. 20. Mosimann W. Anti-infectious chemotherapy in pregnancy. Scweiz Med Wochenschr 1975;105(9):257-63.
Pharmacokinetics
Absorption of spiramycin occurs quickly, but not completely, with great variability (from 10 to 60%).
After oral administration of 6 million IU of spiramycin, Cmax in plasma is about 3.3 mcg/ml.
Spiramycin does not penetrate into the cerebrospinal fluid, but diffuses into breast milk. Penetrates the placental barrier (the concentration in the fetal blood is approximately 50% of the concentration in the maternal blood serum). Concentrations in placental tissue are 5 times higher than corresponding concentrations in serum.
Distribution volume: approximately 383 liters.
The drug penetrates well into saliva and tissues (concentration in the lungs - from 20 to 60 mcg/g, tonsils - from 20 to 80 mcg/g, infected sinuses - from 75 to 110 mcg/g, bones - from 5 to 100 mcg/g ). 10 days after the end of treatment, the concentration of the drug in the spleen, liver and kidneys ranges from 5 to 7 mcg/g.
Plasma protein binding is low (approximately 10%).
Spiramycin is metabolized in the liver to form active metabolites with an unknown chemical structure.
It is excreted mainly in bile (concentrations are 15–40 times higher than in serum). About 10% of the administered dose is excreted by the kidneys.
T1/2 after taking 3 million IU of spiramycin is approximately 8 hours. It may be prolonged in elderly patients. In patients with impaired renal function, no dose adjustment of spiramycin is required.
Instructions for use Spiramycin-Vero (Method and dosage)
Taken orally. Adults—2-3 tablets. 3 million IU per day, divided into 2-3 doses. The maximum daily dose is 9 million ME.
To prevent meningitis caused by meningococcus, take 1 tablet (3 million ME) per day for 5 days.
IV drip only for adults: the contents of the bottle are dissolved in 4 ml of water for injection + 100 ml of 5% dextrose solution .
For pneumonia , 1.5 million IU is prescribed every 8 hours.
Instructions for use of Spiramycin-Vero contain information that if renal function is impaired, dose adjustment is not carried out.
Indications for the drug Spiramycin-vero
bacterial infections caused by sensitive microorganisms: acute community-acquired pneumonia (including atypical pneumonia caused by Mycoplasma, Chlamydia, Legionella), exacerbation of chronic bronchitis; sinusitis, tonsillitis, otitis media; osteomyelitis, arthritis; extragenital chlamydia, prostatitis, urethritis of various etiologies; sexually transmitted diseases;
skin infections: erysipelas, infected dermatoses, abscess, phlegmon (including in dentistry);
toxoplasmosis, incl. during pregnancy;
prevention of meningococcal meningitis among persons who were in contact with patients no more than 10 days before hospitalization;
prevention of acute articular rheumatism;
treatment of bacterial carriage of whooping cough and diphtheria pathogens.
Indications for use
- toxoplasmosis (including during pregnancy);
- community-acquired pneumonia (chlamydial, mycoplasma and caused by aerobic gram-negative bacteria);
- exacerbation of chronic bronchitis ;
- rheumatism, unspecified;
- acute bronchitis ;
- acute tonsillitis , sinusitis , otitis ;
- osteomyelitis;
- sexually transmitted diseases;
- urethritis;
- skin infections;
- prevention of meningitis caused by meningococcus (in persons who had contact with patients);
- bacteria carriage of whooping cough and diphtheria .
Side effects
From the gastrointestinal tract: nausea, vomiting, diarrhea and very rare cases of pseudomembranous colitis (less than 0.01%). Isolated cases of ulcerative esophagitis and acute colitis have been described. The possibility of developing acute damage to the intestinal mucosa in patients with AIDS when using high doses of spiramycin for cryptosporidiosis has also been noted.
From the peripheral system and central nervous system: transient paresthesia.
From the liver: in very rare cases (less than 0.01%) - changes in liver function tests and the development of cholestatic hepatitis.
From the hematopoietic organs: very rare cases (less than 0.01%) of the development of acute hemolysis (see “Contraindications” section “With caution”) and thrombocytopenia.
From the cardiovascular system: possible prolongation of the QT interval on the electrocardiogram.
Hypersensitivity reactions: skin rash, urticaria, itching. Very rarely (less than 0.01%) - angioedema, anaphylactic shock.