1.General information
The term “acidosis” can be translated from Latin as “total acidification.” This is the name given to the decrease in the total hydrogen index (pH) in the body relative to its standard, natural values: 7.37 - 7.44. Maintaining the acid-base balance within these limits is one of the most important elements of homeostasis (constancy of internal conditions), since its strictly defined and fairly narrow range is necessary for the effective occurrence of all biochemical and neurotransmitter reactions, digestive processes, the functioning of symbiotic microflora, and many others.
Acidosis accompanies many diseases, pathological processes and conditions in which the circulation and removal of organic acids from the body is disrupted. The range of such conditions is very wide - from pregnancy and long-term malnutrition to severe infections and endocrine-metabolic disorders (for example, diabetes mellitus). Thus, acidosis is not an independent disease; This is a secondary syndrome that develops due to certain pathogenetic patterns.
A must read! Help with treatment and hospitalization!
Non-gas acidosis
Non-gas acidosis develops through three main mechanisms:
- excessive formation of acidic metabolites in the body, their accumulation and shift in blood pH;
- violation of the excretion of metabolites (they are formed in normal quantities, but cannot leave the body, so they accumulate in it);
- the entry from outside of substances that shift the pH of the blood in quantities exceeding the capabilities of the body’s excretory system.
The first mechanism is most often activated in diabetes mellitus. Against the background of impaired glucose metabolism, lactic acid and ketone bodies accumulate in the body.
Impaired elimination is most often associated with renal failure or hyperaldosteronism: the kidneys lose the ability to remove excess hydrogen ions from the blood.
The introduction of certain drugs into the body can result in acidosis. It is caused by severely exceeding the dose of medication (for example, a suicide attempt) or taking medications against the background of impaired function of the excretory system. Acidosis is often provoked by acetylsalicylic acid taken for a long time in high doses.
2. Reasons
The main reasons for the shift in acid-base balance towards acidification include:
- smoking, drinking alcohol;
- malnutrition (regardless of reasons);
- insufficient fluid intake and dehydration (dehydration) of any other origin;
- intoxication with impaired digestive function of the gastrointestinal tract;
- carrying a pregnancy;
- metabolic disorders;
- cancer;
- hypoxia of any origin;
- a number of kidney diseases.
Visit our Nephrology page
3. Symptoms and diagnosis
Since acidosis occurs against the background and as a result of some pathology, and with its significant severity or long-term course, in most cases the clinical picture of the underlying disease (condition) dominates.
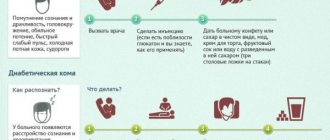
In mild forms, acidosis can manifest itself as weakness, fatigue, nausea, and vomiting. In more severe cases, the so-called Kussmaul breathing (deep, noisy), blood pressure rises, heart rhythm is disrupted. If the pathological “vicious circle” causing acidosis is not interrupted at this stage, then very serious depression of the central nervous system may occur (up to confusion and loss of consciousness) or hypovolemic shock may develop (a life-threatening condition caused by a sudden rapid reduction in the volume of circulating blood and, accordingly, deficiency of tissue oxygenation). A further shift in pH to the acidic side (to 7.1 - 7.2 and below) inevitably leads to coma and then death.
Since the symptoms described above are mainly nonspecific (even in aggregate), acidosis and the degree of its severity are established by laboratory tests of blood and urine (clinical analysis, analysis of gas composition, serum analysis of electrolytes, etc.). It is much more important to identify not the acidosis itself, but its causes, and this must be done as soon as possible, since with some diseases leading to acidosis the patient’s condition can worsen to critical very quickly.
About our clinic Chistye Prudy metro station Medintercom page!
MECHANISM OF DEVELOPMENT AND TREATMENT OF ACIDOSIS IN PATIENTS WITH UREMIA
Endogenous formation of acids
Fats and carbohydrates are metabolized to produce water and carbon dioxide, which is eliminated through the lungs.
As a result of protein metabolism, in addition to carbon dioxide, various non-volatile acids are formed, which are normally excreted by the kidneys. Catabolism of sulfur-containing amino acids, cysteine and methionine, leads to the formation of 40 - 70 mmol of sulfuric acid per day. As a result of the metabolism of cationic amino acids, histidine, lysine, arginine, 138 mmol of free anions are released per day. At the same time, the catabolism of anionic amino acids causes the intake of 100 mmol of bases per day. In addition, food contains organic anions, such as acetate, citrate, lactate and others, which are metabolized to form bicarbonate in an amount of 60 mmol/day. In general, protein metabolism leads to the formation of about 500 mmol of acids, which are normally excreted by the kidneys. High consumption of meat products leads to an increase in the production of acids and acid-forming cationic amino acids. On the contrary, plant foods contain large amounts of organic bicarbonate-forming anions, such as citrate, lactate, etc. Thus, the daily excretion of acids can vary significantly depending on the diet. Renal failure leads to acid retention and a decrease in plasma HCO-3 and total CO2 levels. With moderate acidosis, a decrease in the level of HCO-3 occurs in parallel with an increase in the content of chloride anions, resulting in the formation of hyperchloremic acidosis. As renal failure progresses, the concentration of unmeasured anions increases and acidosis develops with an increased anion gap. Hyperventilation and limited supply of bases from bone tissue partially compensate for the metabolic acidosis of uremia. Measuring the pH of the extracellular fluid allows us to judge the acid-base state inside the cell, since the pH of the intracellular fluid, both in the interdialytic period and during dialysis, changes similar to the change in plasma pH. Possible complications of acidosis in patients with uremia Cardiovascular system
A decrease in pH from 7.4 to 7.2 leads to an increase in the secretion of norepinephrine, which causes tachycardia, arrhythmia, and ventricular fibrillation. Hydrogen ions disrupt the transport of calcium ions inside the myocardial cells, as a result of which its contractile function suffers. Norepinephrine also makes it difficult to transport calcium into the cell. At a pH in the range of 7.4 - 7.2, the negative inotropic effect of acids and the positive inotropic effect of catecholamines compensate each other, and myocardial contractility remains virtually unchanged. Only when the pH drops below 7.2 does the negative inotropic effect predominate. Based on this, it can be assumed that acute or chronic heart disease makes the myocardium more susceptible to the effects of acidosis, and beta blockers and calcium antagonists may enhance the negative inotropic effect of acidosis. Gastrointestinal tract
As in the myocardium, in the muscle cells of the gastrointestinal tract, acidosis leads to a decrease in calcium content, as a result of which muscle tone and contractility decrease.
In vitro, an acidic environment reduces the sensitivity of taenia coli to cholinergic stimulation. Electrolytes
As acidosis increases, protons compete with calcium for protein binding sites, which leads to an increase in the content of ionized calcium in the plasma. A decrease in pH for every 0.1 leads to an increase in potassium concentration by 0.6 μeV/L.
Thus, correction of hyperkalemia by protein restriction also leads to a decrease in the degree of acidosis. In renal failure, especially associated with diabetic nephropathy, a decrease in renin and aldesterone levels is often observed. Hyperkalemia in the setting of diabetes may be a particularly serious problem because ketoacidosis further lowers pH and hypoaldosteronism reduces potassium transport into the cell. Protein metabolism
Acidosis increases protein catabolism without affecting their synthesis, which forms a negative nitrogen balance.
An excess of protein breakdown over their synthesis by only 2% will lead to a loss of protein mass - 168 g/month or 2 kg per year. In patients on dialysis, when the concentration of HCO3 decreased below 22 mmol/l before the dialysis procedure, a decrease in the content of valine in muscle tissue was noted. It is unclear what degree of acidosis is acceptable and what pH and bicarbonate levels must be maintained to eliminate or minimize negative nitrogen balance in uremia. Growth
Studies in patients with renal tubular acidosis (RTA) and experimental data indicate the effect of acidosis on growth rate.
Taking bicarbonate during PCA increases the growth rate. Conflicting data have been obtained on the effect of uremia on the level and activity of growth hormone in uremia. Bones
The bicarbonate content of bones exceeds 8000 mmol, their total buffering capacity is about 25,000 mmol.
In patients with acidosis on dialysis, about 22.4% of the bicarbonate contained in the bones is lost within 2.7 years. Unfortunately, there are no data obtained during 5-10-year or longer follow-up. But if we assume that the bone buffer neutralizes only 20 mmol of acid per day, then the entire buffer capacity of bone tissue in the absence of replenishment will be used up within 3.5 - 4 years. The effect of acidosis on bone depends on the cause of the acidosis and the level of calcium intake and excretion. Treatment of acidosis associated with uremia
Compensatory mechanisms make it possible for many years to neutralize many of the consequences of moderate acidosis at a pH of at least 7.25. At a pH above 7.25, severe complications such as cardiovascular disorders and growth retardation are not observed. The threat of complications occurs at a pH below 7.25 - 7.20. Currently, the most studied and obvious is the effect of acidosis on electrolyte balance and protein metabolism. Maintaining a high serum bicarbonate level (29.1 mmol after dialysis) leads to improved appetite and increased muscle mass. In patients not on dialysis, protein restriction reduces acid intake, and protein-rich foods increase acid excretion by the kidneys. However, protein restriction is not recommended for dialysis patients because it reduces serum albumin and increases mortality. Due to the high content of organic acids in foods of plant origin, the severity of acidosis may be reduced. However, these foods are usually rich in potassium, which is undesirable as kidney failure progresses. In addition, potassium aggravates the severity of acidosis. Calcium carbonate and calcium acetate, the most commonly used phosphate binders, reduce the severity of acidosis in dialysis patients. In the preuremic stage, under the influence of calcium carbonate, the excretion of acids decreases. And finally, far from the last place in the correction of acidosis is the use of bicarbonate, even if it causes gastrointestinal discomfort. The maximum level of pH and bicarbonate concentration in the dialysate is limited by the risk of formation of insoluble calcium carbonate and clogging of the dialysis machine pipes. Overcorrection of acidosis results in metabolic alkalosis at the end of the dialysis procedure. Since the bicarbonate concentration during the interdialysis interval decreases by 6–8 mmol/l, in order for its concentration before the dialysis procedure to correspond to 20–22 mmol/l, at the end of the procedure the serum bicarbonate concentration should reach 28–30 mmol/l.
Literature:
Bommer J, Keller C, Gehlen F, et al. Clin Nephrology 1996;46:280–5.