Detailed description of the study
Inflammation is a protective reaction of the body, which is aimed at limiting and neutralizing damage, the action of a foreign agent, etc. Normally, inflammation is a physiological reaction. However, excessive inflammation can lead to metabolic and hemodynamic disturbances that are harmful to the body, which contribute to the development of severe and protracted diseases.
This comprehensive study quantifies some of the key cytokines—proteins involved in inflammation.
Interleukin 6 (IL-6, IL-6) refers to pro-inflammatory cytokines, i.e. to proteins that increase inflammatory activity. The source of IL-6 in the body is various cells: T-lymphocytes, monocytes, endothelial cells, etc.
Interleukin 6 stimulates the formation of acute-phase proteins - haptoglobin, C-reactive protein, haptoglobulin, etc. - which in turn contribute to an increase in the inflammatory process. IL-6 also regulates the immune response and is involved in the activation of lymphocytes, which trigger the formation of currently required immunoglobulins, or antibodies.
Interleukin 10 (IL-10, IL-10) is a cytokine with pronounced anti-inflammatory properties. IL-10 plays a large role in limiting the inflammatory process, thereby preventing excessive tissue damage. The action of IL-10 helps to reduce the synthesis of other inflammatory cytokines, including TNF-alpha. Dysregulation of interleukin 10 is associated with the development of autoimmune and infectious diseases, as well as inflammation of the vascular endothelium, which can aggravate the course of diabetes mellitus, dyslipidemia - atherosclerosis - and heart pathologies.
Tumor necrosis factor-alpha (TNF-alpha) is one of the key multifunctional proinflammatory cytokines that is predominantly produced in macrophages. Under physiological conditions, it regulates many processes: lipid metabolism; blood clotting; cell division, maturation and death.
Serum TNF-alpha levels may be elevated in a variety of autoimmune, rheumatologic, and infectious diseases. This cytokine also aggravates the course of chronic inflammation in the presence of malignant tumors.
A comprehensive determination of the above cytokines may be relevant for additional diagnosis of the following pathologies:
- Systemic lupus erythematosus and other rheumatological diseases;
- Many inflammatory diseases of an infectious or autoimmune nature;
- "Cytokine storm"
The latter syndrome is the most relevant indication. "Cytokine storm" is an umbrella term for the body's overactive inflammatory response, which is accompanied by the production of excess cytokines (including IL-6, IL-10, and TNF-alpha).
The causes of a “cytokine storm” include:
- Severe viral infections: influenza, new coronavirus infection COVID-19, etc.;
- Infectious toxic shock (sepsis);
- Malignant neoplasms;
- Transplantation of organs and tissues.
It is recommended to take an analysis to determine these cytokines in the blood only if there is indications from the attending physician.
Chemokines
Chemokines stimulate chemotaxis - the directed movement and movement of leukocytes. Chemokines are produced by leukocytes, platelets, epithelial and endothelial cells. These cells are located in places where pathogens most often penetrate (skin, mucous membranes, blood vessels) and, through chemokines, call leukocytes to help quickly neutralize foreign antigens. On the downside, the human immunodeficiency virus has adapted to chemokine receptors on the surface of T-lymphocytes, using them to penetrate the cell.
References
- Shipilov, M.V. Molecular mechanisms of the “cytokine storm” in acute infectious diseases. General Medicine, 2013. - No. 1.
- Nedomolkina, S.A., Velikaya, O.V., Zoloedov, V.I. Cytokine status in patients with chronic obstructive pulmonary disease and type 2 diabetes mellitus. Kazan medical journal, 2022. - No. 2.
- Iyer, S., Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol., 2012. - Vol. 32(1). - P. 23-63.
- Chu, W. Tumor necrosis factor. Cancer Lett., 2013. - Vol. 328(2). — P. 222-225.
- Ligong, L., Zhang, H., Danielle, J. Dauphars, You-Wen He, A Potential Role of Interleukin 10 in COVID-19 Pathogenesis, Trends in Immunology, 2022. - Vol. 42(1). — P. 3-5.
- Giovannini, S., Onder, G., Liperoti, R. et al. Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J Am Geriatr Soc., 2011. - Vol. 59(9). — P. 1679-1685.
Colony-stimulating factors
Produced by endothelial cells, fibroblasts, macrophages, mast cells and T helper cells. Designed to stimulate hematopoiesis - the growth of blood cells. Highlight:
Granulocyte colony-stimulating factor (G-CSF) stimulates the growth of neutrophil precursors.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulates the growth of monocytes, neutrophils, eosinophils and basophils, and activates macrophages.
Macrophage colony-stimulating factor (M-CSF) stimulates the growth of monocyte precursors.
| Cytokines | Producer cells | Functions |
Transformative growth factors | ||
| Transforming growth factor alpha | Macrophages, monocytes, epithelial cells, bone marrow cells | Stimulates the growth and development of immune cells. Stimulates mucus production. |
| Transforming growth factor beta | B lymphocytes, macrophages, mast cells | Suppresses the growth of lymphocytes, cancels the effects of many cytokines, switches to the synthesis of IgA. Promotes tissue healing and connective tissue growth at the site of inflammation. |
The skin I live in
Figure 1. Skin structure.
website bono-esse.ru
Skin is the largest human organ. Taking into account subcutaneous fat tissue, it amounts to up to 17% of body weight.
The skin consists of three main parts (Fig. 1).
- The epidermis is precisely that superficial part of the skin, accessible to our eyes, that peels off after a sunburn or forms calluses after mechanical stress. Thanks to the epidermis, the body's protective barrier is formed from pathogenic bacteria, fungi, viruses, temperature, ultraviolet radiation, water loss and mechanical damage. The epidermis is formed by several types of cells, but the most common type (up to 90% of the total) is keratinocytes . They are called so because they produce the structural protein keratin in huge quantities. The epidermis consists of five layers: basal, spinous, granular, shiny and horny (Fig. 2). Keratinocytes of the basal layer actively divide, displacing older cells to the surface of the skin. On the way to the surface, the cells differentiate - they flatten, become keratinized (produce huge amounts of keratin) and, gradually dying, lose their nuclei. At different stages of differentiation, keratinocytes look different under a light microscope; their appearance gives the name to the layers of the epidermis: basal - cells located on the basement membrane; spinous - cells with spine-like processes; granular - cells containing accumulations of proteins and fats in the form of grains in the cytoplasm; shiny - cells with shiny protein eleidin; horny - horny plates (nuclear-free, keratinized cells). melanocyte cells , which synthesize the pigment melanin, which is responsible for protecting the skin from UV rays, and immune cells (primarily dendritic cells), which are the first to encounter infectious and non-infectious agents that have penetrated the skin.
- The dermis is the deeper part of the skin, separated from the epidermis by a thin layer of basement membrane, formed by blood vessels and structural proteins (primarily collagen and elastin). The dermis consists of two layers: the papillary layer, rich in nourishing vessels, and the reticular layer, rich in proteins responsible for the strength and elasticity of the skin. The main and most numerous cells of this part of the skin are fibroblasts , which secrete collagen and elastin. Also present in the dermis are single melanocytes and some immune cells, primarily macrophages .
- Hypodermis is subcutaneous fatty tissue formed by adipocyte . Since fat is an excellent heat insulator and also an energy depot, the hypodermis performs thermoregulatory and energy functions. In addition, the fiber layer serves as additional protection for underlying tissues from damage.
Figure 2. Five layers of the epidermis.
website
Also in the skin there are sebaceous and sweat glands that open their ducts on its surface, hair follicles, nerve fibers and nerve endings.
Innate lymphoid cells type 2 (ILC2) in the regulation of allergic reactions
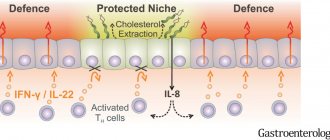
ILC2s, representing the innate immune system, are one of the main participants in the development of allergic reactions in various organs. Now these cells are receiving much attention as analogues or unique counterparts of Th2, which also synthesize the cytokines IL-4, IL-5, IL-9, IL-13 and amphiregulin in response to the action of TSLP, IL-25 and IL-33 [23–25 ]. Actually, this is why the name “type 2 immune response” is adopted, and not the Th2 response, as it was previously designated. Among ILC2, additional subpopulations are currently being identified based on their ability to respond to IL-25 and IL-33. The subpopulation that responds to IL-33 are quiescent cells called natural ILC2s (nILC2s), while the cells that respond to IL-25 are called inflammatory ILC2s (iILC2s) [26]. In addition, a subpopulation of ILC2 that produces IL-10 is isolated [27]. Perhaps they normally function to limit the excessive synthesis of proinflammatory cytokines during the development of a response to parasite invasion.
ILC2s are involved in the immunopathogenesis of allergic rhinitis and are found in large numbers in nasal polyp tissue. Their number increases in the peripheral blood of patients, and these cells appear in the nasal mucosa with the development of allergic rhinitis. At the same time, successful allergen-specific immunotherapy leads to a decrease in the number of ILC2 in patients [28, 29]. The use of anti-cytokine therapy using monoclonal antibodies against IL-5 gives a good therapeutic effect in polypous rhinosinusitis [30]. ILC2s serve as an important component of AD development. In patients with asthma, an increase in the number of ILC2s in the peripheral blood, activation of ILC2s in lung tissue, and active synthesis of cytokines characteristic of type 2 immune response were noted [31]. In particular, ILC2 secrete IL-13, which stimulates the migration of activated DCs to draining lymph nodes, where they trigger Th2 differentiation [32]. In AD patients, an encounter with an allergen causes a sharp increase in the number and activation of ILC2s secreting IL-5 and IL-13 [33]. The levels of ILC2 and IL-33 in bronchoalveolar lavages of patients correlated with the severity of asthma [34].
A thin scar on your favorite skin
Despite the fact that every day we are learning more and more about the contribution of the immune system to the development of psoriasis, it is not yet possible to completely cure this disease. Even the most successful treatment methods can only achieve periods of remission - but sooner or later the plaques reappear on the skin. The search for the reason for this state of affairs led researchers to carefully study the keratinocytes in the area of the skin where the plaque used to be. It turned out that, despite the visual improvement characteristic of remission, the disappearance of the characteristic histological symptoms of psoriasis and “extra” immune cells from the skin, the expression of some genes in keratinocytes remains altered. This change has been called a molecular scar, and it may be the reason why the disease comes back again and again. Here are some genes whose activity never returns to normal: the MMP9 gene, which encodes an enzyme that breaks down different types of collagen and gelatin in the skin, helping skin cells to be more mobile; the WNT5A gene, encoding an important regulatory protein that monitors the proper development of skin and hair follicles, and can also regulate skin color by influencing melanocytes; genes of various cytokines (CCL2, CXCR4, CCL18, LTB) and several hundred more genes [16]. Perhaps studying these genes will tell us something new about the role of keratinocytes in the development and recurrence of psoriasis.
Introduction
According to modern concepts, psoriasis is a multifactorial disease that develops in people with a genetic predisposition under the influence of the external environment. Until now, the pathogenesis of psoriasis has not been fully studied, but over the past decades, scientists have made great progress in its research: they have found explanations for previously observed clinical phenomena, established mechanisms of interaction between cells of the immune system and skin cells, and identified the relationship between the pathogenesis of psoriasis and other diseases. This article focuses on the pathogenesis of psoriasis; the genetic and epigenetic aspects of the disease will be described separately.
As already mentioned in the introductory article [1], with psoriasis, patients with psoriasis develop raised, reddened areas on the skin - plaques. Their appearance is associated with two processes: inflammation in the skin and hyperplasia (overgrowth) of the epithelium. Apparently, the reason for this is misregulation of the interaction of the immune system with skin cells [2]. Therefore, in order to understand the pathogenesis of psoriasis, you first need to learn a little about the structural features of the skin and the principles of operation of the immune system.