Story
In the 13th century, the famous Spanish scientist Raymond Lull discovered diethyl ether. Its properties were described in 1540 by the equally famous scientist Paracelsus. In 1846, ether was first tried as an anesthesia. The operation performed by the American doctor D. Warren using vapors of this substance was successful. The inventors of anesthesia are considered to be the dentist W. Morton and his mentor, the physician and chemist C. Jackson.
Methods for obtaining ether in the 16th century were described by Valerius Cordus, a famous botanist and pharmacist originally from Kassel. Since the beginning of the 18th century, an alcohol-ether mixture has been used as a sedative - this was the proposal of Friedrich Hoffmann. In 1796, the St. Petersburg pharmacist Thomas Lovitz obtained pure diethyl ether, the formula of which, by the way, has two versions (more on this a little later). But the principle of action of the mentioned substance on the human body was unveiled by the English physicist M. Faraday, after which even a scientific article devoted to this topic was published in 1818.
Side effects of the drug Ethyl ether
Irritation of the respiratory tract may be accompanied at the beginning of anesthesia by reflex changes in breathing, up to laryngospasm and apnea. A sharp increase in blood pressure and tachycardia may be observed (due to an increase in the content of norepinephrine and epinephrine in the blood), especially during periods of excitement. In the postoperative period, vomiting and respiratory depression are often observed. Due to the irritating effect on the mucous membranes of the respiratory tract, bronchitis and pneumonia may develop in the postoperative period.
Characteristic
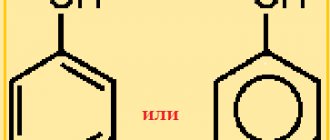
What is called simple ether? This is an organic compound whose molecules consist of two hydrocarbon radicals and an oxygen atom. The most important is simple diethyl ether, the formula of which is as follows:
(C2H5)2O or C4H10O.
It is a colorless, transparent, very mobile volatile liquid with a peculiar odor and pungent taste.
When exposed to light, air, heat and moisture, ether can decompose, forming toxic aldehydes, peroxides and ketones that irritate the respiratory tract.
At a water temperature of 20 degrees, it dissolves by 6.5%. Mixes well with fatty and essential oils, benzene and alcohol, regardless of the ratio.
The ether itself, however, like its vapors, is highly flammable. In a certain proportion with oxygen or air, diethyl ether vapor used for anesthesia is explosive.
Overdose
Uncontrollable craving for diethyl ether persists for several years and usually ends with a complete transition to harder drugs. Gradually, the usual dosage becomes insufficient and the addict is forced to increase the volume of inhaled vapor in order to achieve euphoria. All this usually leads to an overdose.
Signs of diethyl ether intoxication:
- severe cramps,
- drop in blood pressure,
- suffocation,
- excited state
- feeling of fear,
- panic.
Note! In case of an overdose of ethers, dysfunction of the respiratory and cardiovascular systems with possible death cannot be ruled out.
The only way to help a drug addict overdose on an essential drug is to seek professional medical help. Do not try to induce vomiting in a drug addict, do not give him any medications, wait for the doctor to arrive.
Chemical properties
Diethyl ether has all the chemical properties of ethers. So, let's look at this issue in more detail. This is a fairly inert substance. The main difference from esters is the lack of hydrolysis, although there are exceptions. In the cold it does not react with phosphorus chloride, sodium metal and many dilute mineral acids. Despite this, concentrated acids (sulfuric and hydroiodic) decompose these esters even at low temperatures, and heated metallic sodium breaks them down.
An ester with lone pairs of electrons interacts with a proton of a strong acid, resulting in the formation of an unstable oxonium compound:
- Acidolysis. Sulfuric and hydroiodic acids, as well as FeCl3 in acetic anhydride, are capable of breaking down ethers. The chemical reaction looks like this: CH3—CH2—O—CH2—CH3 + HJ → CH3—CH2—OH + J—CH2—CH3.
— Metalation reaction, called the Shorygin reaction. Heated sodium metal splits diethyl ether: C2H5–O–C2H5 + 2Na → C2H5ONa + C2H5Na
— Relative chemical inertness does not prevent ethers from forming peroxides when stored in air, which often leads to explosions at the end of distillation.
Effect on the body
Ether is a drug that provokes a change in human consciousness. The hallucinations that arise seem so real that the addict tries to interact with them: this can be dangerous not only for him, but also for the people around him.
Hallucinations can be not only visual, but also auditory, tactile, and olfactory.
With constant use of the drug ether, physical dependence with its inherent withdrawal syndrome does not form. However, people who use the substance for a long time develop persistent mental dependence. It is IMPOSSIBLE to overcome it on your own without the help of professional narcologists and psychologists .
Consequences of inhaling ether:
- irritation of mucous membranes,
- frequent bronchitis and pneumonia,
- degradation of a drug addict,
- neuropsychiatric diseases,
- toxic liver damage,
- kidney dysfunction.
Ether dependence is accompanied by pronounced dissociation. A drug addict loses interest in his hobbies; all his thoughts and actions are focused on searching for and using drugs. In its absence, there is increased irritability, severe headaches and outbursts of aggression.
Diethyl ether: physical properties
The peculiar smell and low boiling point of ethers are evidence of weak intermolecular influence, and this indicates low polarity and the absence of prerequisites for the formation of hydrogen bonds. Unlike alcohols, ethers have stronger electron-donating properties, which is confirmed by the value of ionization potentials. The enhancement of these features is associated with the positive inductive effect of the group of atoms resulting from alkanes when the hydrogen atom is removed.
The boiling point of diethyl ether is 35.6 degrees Celsius (this is much lower than that of isomeric alcohols), and the freezing point is 117 °C. Ethers are almost immiscible with water. The explanation for this is quite simple: they are not able to form hydrogen bonds, since their molecules do not have polar bonds. Diethyl ether, whose density relative to hydrogen oxide is 0.714, is also poorly soluble in water. One of the features of the substance in question is its tendency to electrify. The likelihood of static electricity discharges is especially high when pouring or draining chemical compositions, which can result in ignition. Ether vapors form explosive mixtures with air, which is 2.5 times lighter. The lower explosive limit is 1.7%, and the upper limit is 49%. When working with ether, one should not forget that its vapors tend to spread over long distances without losing their ability to burn. So the main precaution is to stay away from open flames and other sources of ignition.
Ether is a low-active compound, several times less reactive compared to alcohols. Remarkably dissolves most organic substances, due to which it is used as a solvent. Diethyl ether is no exception. Its physical properties, as well as its chemical ones, make it possible to use it in medicine and in production.
Narcotic effect
Drug addicts inhale ether vapor in relatively small volumes to get a feeling of high and excitement. The effect of inhaling vapors can be compared to drinking alcohol. Euphoria occurs against the background of a gradually advancing anesthetic. The peak of narcotic intoxication occurs within 1-2 minutes after inhalation, but the effect is relatively short-lived and reaches 10-15 minutes.
Ether is a substance that is not dangerous to human health and life, provided that it is used for medical purposes. Its use as a drug provokes deep depression of the central nervous system, while the effect of the ether develops in stages:
| Stage No. 1 - general anesthesia is accompanied by loss of sensitivity, while consciousness remains clear, normal physical indicators are preserved | Stage No. 2 – excitement, increased motor and speech activity, increased blood pressure, appearance of a gag reflex |
| Stage No. 3 – “anesthesia” The central nervous system is depressed, signs of an excited state disappear, muscle tone is restored and blood pressure returns to normal | Stage No. 4 - agonal is observed during intoxication and is accompanied by shallow breathing, a very weak pulse and greatly dilated pupils |
The agonal stage ends in death if the substance abuser does not receive professional help in a timely manner.
Note! During a trip, the addict is not able to control his own actions, so it is not customary to use diethyl ether alone.
A few hours after consumption, the ether vapors begin to evaporate. The narcotic effect gradually wears off, ending with deep and long sleep. The feeling of detachment from the real world and surrounding reality usually continues for another 1-2 days, after which the drug addict begins to need another dose.
Preparation of diethyl ether
Ethers do not occur in nature; they are obtained synthetically. Under the influence of acid catalysts on ethyl alcohol at elevated temperatures, diethyl ether is obtained (the formula is indicated above). The easiest way to obtain this substance is by distilling a mixture consisting of sulfuric acid and alcohol. To do this, it must be heated to 140-150 degrees Celsius. We will need ethyl alcohol and sulfuric acid (in equal proportions), pipettes, test tubes and gas outlet tubes.
So, after the equipment and reagents are prepared, you can begin the experiment. Pour 2-3 ml of a mixture of alcohol and acid into a test tube (it must be dry) and heat it slowly. As soon as boiling begins, the burner is removed, and 5 to 10 drops of ethyl alcohol are added to the hot mixture using a pipette along the wall of the test tube. The reaction that occurs looks like this:
- CH3—CH2—OH (ethylsulfuric acid) + H2S04 CH3—CH2—OSO3H + H2O;
- CH3—CH2—OSO3H + CH3—CH3—O;
- CH3—CH2—O—CH2—CH3 (diethyl ether)+ H2SO4.
The formation of diethyl ether is indicated by the appearance of an odor.
Ether use by drug addicts
Drug addicts (substance abusers) inhale ether in relatively small volumes to obtain a feeling of euphoria and excitement. After inhaling the substance, a person becomes overly active and talkative.
It is almost impossible to obtain medical (diethyl, for anesthesia) ether for drug addicts whose activities are not directly related to working with this substance. Therefore, the drug has to be obtained through people working in the medical field and having access to anesthesia and anesthesia (anesthesiologists). Less commonly, drug addicts obtain the substance through laboratory assistants who use or receive the substance during various chemical reactions, or through employees of chemical industry enterprises.
Drug addiction?
Get a consultation now
Use in medicine
Doctors use diethyl ether as a general anesthetic drug. The properties of this substance do not allow it to be used in operations where power tools are used, since it is highly flammable and can explode when combined with air. Diethyl ether is widely used in surgery, where it is used for inhalation anesthesia. In dentistry, they are used to treat dental root canals and carious areas, thus preparing the oral cavity for filling.
Pharmacology
The ether easily penetrates the blood-brain barrier, quickly reaching the deep structures of the brain when administered by inhalation. In this case, the substance affects tissues unevenly, affecting mainly neurons. The product is also used for pain relief in dentistry and “distraction” therapy (local irritation by rubbing).
Mechanism of action
According to its pharmacological properties, ester is a CNS depressant, that is, it inhibits its functions. The main targets are the membranes of the processes of nerve cells that transmit electrical impulses. The substance reversibly disrupts the ultrastructure and interaction of neurons in the deep parts of the brain.
Inhibition of interneuronal transmission can lead to a compensatory increase in the activity of other areas. The patient temporarily loses consciousness, all types of sensitivity and reflexes. Mutually enhances the effect of various CNS depressants; the most dangerous are combinations with analeptics, psychostimulants, alcohol, and drugs.
How does addiction develop?
In the early stages, drug addicts do not feel any side effects, so they do not worry about their health. You can easily get ether at a pharmacy without a prescription at a reasonable price. It does not take much time to achieve drug intoxication. After inhaling the vapors, a person instantly relaxes, and after 15 minutes loses touch with reality. Therefore, addiction to ether develops instantly, and it is difficult to get rid of it. The dependence is purely psychological. A person thinks that he can always quit. This is self-deception, since at the first attempt to give up ether, withdrawal syndrome develops. The drug addict suffers from terrible headaches, aggression, and insomnia. The state of health returns to normal without the help of doctors within a week. The desire to achieve euphoria again thanks to ether can last for many years.
Treatment for ether addiction
You can only get rid of drug addiction with the help of professionals. It is important to pay attention to socio-psychological rehabilitation and deeply work through the problem with a psychotherapist.
If you find out that a person close to you uses ether or find a strange cap with a clear liquid and a pungent odor in his room, call the Zdravnitsa professional treatment and rehabilitation center at 8 ( 800) 200-27-23 . We work around the clock and anonymously.