L-Thyroxin 75 Berlin Chemi
The use of tricyclic antidepressants with levothyroxine sodium may lead to increased effects of the antidepressants.
Levothyroxine sodium reduces the effect of cardiac glycosides.
When used simultaneously, cholestyramine and colestipol (ion exchange resins) reduce the plasma concentration of levothyroxine sodium by inhibiting its absorption in the intestine. In this regard, levothyroxine sodium must be taken 4-5 hours before taking these drugs.
Colesevelam binds to levothyroxine sodium and thus reduces the absorption of levothyroxine sodium from the gastrointestinal tract. Although no interactions have been observed when levothyroxine sodium was administered at least 4 hours before colesevelam, levothyroxine sodium should be taken at least 4 hours before colesevelam.
When used simultaneously with anabolic steroids, asparaginase, tamoxifen, pharmacokinetic interaction is possible at the level of binding to plasma proteins.
Protease inhibitors (eg, ritonavir, indinavir, lopinavir) may affect the effectiveness of levothyroxine sodium. Close monitoring of thyroid hormone concentrations is recommended. Thyroid-stimulating hormone (TSH) should be monitored in patients taking levothyroxine sodium for at least the first month after starting and/or stopping treatment with ritonavir. If necessary, the dose of levothyroxine sodium should be adjusted.
Phenytoin may affect the effectiveness of levothyroxine sodium due to the displacement of levothyroxine sodium from plasma proteins, which can lead to increased concentrations of free T4 and T3. On the other hand, phenytoin increases the rate of metabolism of levothyroxine sodium in the liver. Close monitoring of thyroid hormone concentrations is recommended.
Levothyroxine sodium may reduce the effectiveness of hypoglycemic drugs. Therefore, frequent monitoring of blood glucose concentrations is necessary from the start of thyroid hormone replacement therapy. If necessary, the dose of the hypoglycemic drug should be adjusted.
Levothyroxine sodium may enhance the effect of anticoagulants (coumarin derivatives) by displacing them from plasma proteins, which may increase the risk of bleeding, such as cerebral hemorrhage or gastrointestinal bleeding, especially in elderly patients. Therefore, regular monitoring of coagulation parameters is necessary both at the beginning and during combination therapy with these drugs. If necessary, the dose of the anticoagulant should be adjusted.
Salicylates, dicumarol, furosemide in high doses (250 mg), clofibrate and other drugs can displace levothyroxine sodium from binding to plasma proteins, which leads to an increase in the concentration of free T4.
Sevelamer and lanthanum carbonate may reduce the absorption of levothyroxine sodium. Tyrosine kinase inhibitors (eg, imatinib, sunitinib) may reduce the effectiveness of levothyroxine sodium. Therefore, at the beginning or end of a course of simultaneous therapy with these drugs, it is recommended to monitor changes in thyroid function in patients. If necessary, the dose of levothyroxine sodium is adjusted. Aluminum-containing drugs (antacids, sucralfate) are described in the literature as potentially reducing the effectiveness of levothyroxine sodium. That's why
It is recommended to take levothyroxine sodium at least 2 hours before using these medications. This recommendation also applies to the use of medications containing iron and calcium salts.
Somatropin, when used simultaneously with levothyroxine sodium, can accelerate the closure of epiphyseal growth plates.
Propylthiouracil, glucocorticosteroids, beta-sympatholytics, iodinated contrast agents and amiodarone inhibit the conversion of T4 to T3. Due to the high iodine content, the use of amiodarone may be accompanied by the development of both hyperthyroidism and hypothyroidism. Particular attention should be paid to nodular goiter with the possible development of unrecognized functional autonomy.
Sertraline, chloroquine/proguanil reduce the effectiveness of levothyroxine sodium and increase the concentration of TSH in the blood plasma.
Drugs that induce hepatic enzymes (eg, barbiturates, carbamazepine) may enhance the hepatic clearance of levothyroxine sodium.
In women using estrogen-containing contraceptives or in postmenopausal women receiving hormone replacement therapy, the need for levothyroxine sodium may be increased.
Consumption of soy-containing products may reduce the intestinal absorption of levothyroxine sodium. Therefore, dosage adjustments may be necessary, especially when starting or stopping consumption of soy-containing products.
With simultaneous use of orlistat and levothyroxine sodium, hypothyroidism may develop and/or a decrease in the control of hypothyroidism may occur. The reason for this may be decreased absorption of iodine salts and/or levothyroxine sodium. Patients taking levothyroxine sodium should consult their doctor before starting orlistat, as it may be necessary to take orlistat and levothyroxine sodium at different times of the day, and the dose of levothyroxine sodium may need to be adjusted. Further monitoring of thyroid function is recommended.
L-Thyroxine 75 Berlin-Chemie tablet 75 mcg cor x100
L-Thyroxine, 75 Berlin-Chemie tablet, 75, µg x100, ATX code: H03AA01 (Levothyroxine sodium) Active substance: levothyroxine sodium Rec.INN registered by WHO
Dosage forms
L-THYROXINE 50 BERLIN-CHEMIE
tab. 50 mcg: 25, 50 or 100 pcs.reg. No.: P N008963 dated 02/28/11 - Indefinitely
tab. 75 mcg: 25, 50 or 100 pcs.reg. No.: LSR-001294/08 dated 02.28.08 - Indefinitely
tab. 100 mcg: 25, 50 or 100 pcs.reg. No.: P N008964 dated 02/28/11 - Indefinitely
tab. 125 mcg: 25, 50 or 100 pcs.reg. No.: LSR-001807/08 from 03/17/08 - Indefinitely
tab. 150 mcg: 25, 50 or 100 pcs.reg. No.: LSR-001484/08 from 03.14.08 - Indefinitely
Release form, composition and packaging
The tablets are white to slightly beige in color, round, slightly biconvex, with a score line on one side and “75” embossed on the other.
1 tab.
levothyroxine sodium 75 mcg
Excipients: calcium hydrogen phosphate dihydrate, microcrystalline cellulose, sodium carboxymethyl starch (type A), dextrin, long-chain partial glycerides.
Clinical-pharmacological group: Thyroid hormone preparation Pharmaco-therapeutic group: Thyroid drug
pharmachologic effect
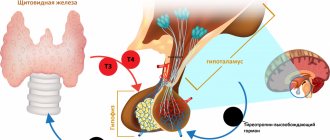
A synthetic preparation of thyroid hormone, a levorotatory isomer of thyroxine. After partial conversion into triiodothyronine (in the liver and kidneys) and passage into the cells of the body, it affects the development and growth of tissues and metabolism.
In small doses it has an anabolic effect on protein and fat metabolism. In medium doses, it stimulates growth and development, increases tissue oxygen demand, stimulates the metabolism of proteins, fats and carbohydrates, and increases the functional activity of the cardiovascular system and central nervous system. In high doses, it inhibits the production of TTRH from the hypothalamus and TSH from the pituitary gland.
The therapeutic effect is observed after 7-12 days, during the same time the effect persists after discontinuation of the drug. The clinical effect for hypothyroidism appears after 3-5 days. Diffuse goiter decreases or disappears within 3-6 months.
Pharmacokinetics
Suction
After oral administration, levothyroxine is absorbed almost exclusively from the upper small intestine. Up to 80% of the dose taken is absorbed. Concomitant food intake reduces the absorption of levothyroxine. Cmax in serum is achieved approximately 5-6 hours after oral administration.
Distribution
Binds to serum proteins (thyroxine-binding globulin, thyroxine-binding prealbumin and albumin) by more than 99%. Approximately 80% of levothyroxine is monodeiodinated in various tissues to form triiodothyronine (T3) and inactive products.
Metabolism
Thyroid hormones are metabolized mainly in the liver, kidneys, brain and muscles. A small amount of the drug undergoes deamination and decarboxylation, as well as conjugation with sulfuric and glucuronic acids (in the liver).
Removal
Metabolites are excreted in urine and bile.
T1/2 is 6-7 days.
Pharmacokinetics in special clinical situations
With thyrotoxicosis, T1/2 is shortened to 3-4 days, and with hypothyroidism it is extended to 9-10 days.
Indications
- hypothyroidism,
- euthyroid goiter,
— as replacement therapy and for the prevention of goiter recurrence after resection of the thyroid gland,
- thyroid cancer (after surgical treatment),
- diffuse toxic goiter: after achieving a euthyroid state with thyreostatics (in the form of combination or monotherapy),
- as a diagnostic tool when performing a thyroid suppression test.
ICD-10 codes
Dosage regimen
The daily dose is determined individually depending on the indications.
L-Thyroxine Berlin-Chemie in a daily dose is taken orally in the morning on an empty stomach, at least 30 minutes before meals, washing down the tablet with a small amount of liquid (half a glass of water) and without chewing.
When carrying out replacement therapy for hypothyroidism, patients under 55 years of age in the absence of cardiovascular diseases L-Thyroxine Berlin-Chemie are prescribed in a daily dose of 1.6-1.8 mcg/kg body weight, patients over 55 years of age or with cardiovascular diseases - 0.9 mcg/kg body weight bodies. In case of significant obesity, the calculation should be made on the “ideal body weight”.
Initial stage of replacement therapy for hypothyroidism
Patients without cardiovascular diseases under 55 years of age Initial dose: women - 50-100 mcg / day, men - 50-150 mcg / day
Patients with cardiovascular diseases or over 55 years of age The initial dose is 25 mcg/day. Increase by 25 mcg at intervals of 2 months until the TSH level in the blood normalizes. If symptoms from the cardiovascular system appear or worsen, adjust the therapy for cardiovascular diseases.
Recommended doses of thyroxine for the treatment of congenital hypothyroidism
Age Daily dose of thyroxine (mcg) Dose of thyroxine per body weight (mcg/kg)
0-6 months 25-50 10-15
6-24 months 50-75 8-10
from 2 to 10 years 75-125 4-6
from 10 to 16 years 100-200 3-4
>, 16 years 100-200 2-3
Indications Recommended doses of L-Thyroxine Berlin-Chemie (mcg/day)
Treatment of euthyroid goiter 50-200
Prevention of relapse after surgical treatment of euthyroid goiter 50-200
In complex therapy of thyrotoxicosis 50-100
Suppressive therapy for thyroid cancer 150-300
Thyroid suppression test 4 weeks before test 3 weeks before test 2 weeks before test 1 week before test
50-75 75-100 150-200 150-200
For precise dosing of the drug, the most appropriate dosage of the drug L-Thyroxine Berlin-Chemie should be used (50, 75, 100, 125 or 150 mcg).
In case of severe long-term hypothyroidism, treatment should be started with extreme caution, with small doses - from 25 mcg / day, the dose is increased to maintenance at longer intervals - by 25 mcg / day every 2 weeks and the level of TSH in the blood is determined more often. For hypothyroidism, L-Thyroxine Berlin-Chemie is usually taken throughout life.
For thyrotoxicosis, L-Thyroxine Berlin-Chemie is used in complex therapy with thyreostatics after achieving a euthyroid state. In all cases, the duration of treatment with the drug is determined by the doctor.
For infants and children under 3 years of age, the daily dose of L-Thyroxine Berlin-Chemie is given in one dose 30 minutes before the first feeding. The tablet is dissolved in water to a thin suspension, which is prepared immediately before taking the drug.
Side effect
When using the drug according to indications in recommended doses under the supervision of a physician, no side effects are observed.
If you are hypersensitive to the drug, allergic reactions may occur.
Contraindications for use
- hypersensitivity to the components of the drug,
- untreated thyrotoxicosis,
- acute myocardial infarction, acute myocarditis,
- untreated adrenal insufficiency.
The drug should be prescribed with caution in case of coronary artery disease (atherosclerosis, angina pectoris, history of myocardial infarction), arterial hypertension, arrhythmia, diabetes mellitus, severe long-term hypothyroidism, malabsorption syndrome (dose adjustment may be required).
Use during pregnancy and breastfeeding
During pregnancy and lactation (breastfeeding), therapy with the drug prescribed for hypothyroidism should continue. During pregnancy, an increase in the dose of the drug is required due to an increase in the level of thyroxine-binding globulin. The amount of thyroid hormone secreted in breast milk during lactation (even when treated with high doses of the drug) is not enough to cause any problems in the child.
The use of the drug in combination with thyreostatic drugs during pregnancy is contraindicated, because Taking levothyroxine may require increasing doses of thyreostatics. Since thyreostatics, unlike levothyroxine, can penetrate the placental barrier, the fetus may develop hypothyroidism.
During breastfeeding, the drug should be taken with caution, strictly in recommended doses under medical supervision.
Use in children
In children, the initial daily dose is 12.5-50 mcg. For a long course of treatment, the dose of the drug is determined from an approximate calculation of 100-150 mcg/m2 of body surface area.
For infants and children under 3 years of age, the daily dose of L-Thyroxine Berlin-Chemie is given in one dose 30 minutes before the first feeding. The tablet is dissolved in water to a thin suspension, which is prepared immediately before taking the drug.
Use in elderly patients The drug should be prescribed to elderly patients with extreme caution and under regular medical supervision.
special instructions
In case of hypothyroidism caused by damage to the pituitary gland, it is necessary to find out whether there is simultaneous insufficiency of the adrenal cortex. In this case, replacement therapy with GCS should be started before treatment of hypothyroidism with thyroid hormones is started in order to avoid the development of acute adrenal insufficiency.
Impact on the ability to drive vehicles and operate machinery
The drug does not affect the ability to drive vehicles and work requiring increased concentration.
Overdose
Symptoms characteristic of thyrotoxicosis: palpitations, irregular heart rhythm, heart pain, anxiety, tremors, sleep disturbance, increased sweating, loss of appetite, weight loss, diarrhea.
Treatment: a reduction in the daily dose of the drug, a break in treatment for several days, and the appointment of beta-blockers may be recommended. After side effects disappear, treatment should be started with caution at a lower dose. Antithyroid drugs are not recommended.
Drug interactions
Levothyroxine enhances the effect of indirect anticoagulants, which may require a reduction in their dose.
The use of tricyclic antidepressants with levothyroxine may result in increased antidepressant effects.
Thyroid hormones may increase the need for insulin and oral hypoglycemic agents. More frequent monitoring of blood glucose levels is recommended during periods of initiation of treatment with levothyroxine, as well as when changing the dose of the drug.
Levothyroxine reduces the effect of cardiac glycosides. With simultaneous use of cholestyramine, colestipol and aluminum hydroxide, they reduce the plasma concentration of levothyroxine by inhibiting its absorption in the intestine.
When used simultaneously with anabolic steroids, asparaginase, tamoxifen, pharmacokinetic interaction is possible at the level of protein binding.
When used simultaneously with phenytoin, salicylates, clofibrate, furosemide in high doses, the content of levothyroxine and T4 unbound to blood plasma proteins increases.
Somatotropin, when used simultaneously with levothyroxine, can accelerate the closure of epiphyseal growth plates.
Taking phenobarbital, carbamazepine and rifampicin may increase the clearance of levothyroxine and require an increase in dose.
Estrogens increase the concentration of the thyroglobulin-bound fraction, which may lead to a decrease in the effectiveness of the drug.
Amiodarone, aminoglutethimide, PAS, ethionamide, antithyroid drugs, beta-blockers, carbamazepine, chloral hydrate, diazepam, levodopa, dopamine, metoclopramide, lovastatin, somatostatin affect the synthesis, secretion, distribution and metabolism of the drug.
Storage conditions and periods
The drug should be stored out of the reach of children at a temperature not exceeding 25°C. Shelf life – 2 years.
Conditions for dispensing from pharmacies
The drug is available with a prescription.