Pharmacological properties
Pharmacodynamics
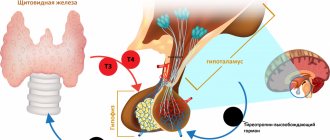
Mechanism of action. Synthetic levothyroxine, which is contained in the preparation L-Thyroxine 50, 75, 100, 125 and 150 Berlin-Chemie, is identical in biological activity to the natural thyroid hormone. There is no difference between endogenously formed and exogenous levothyroxine for the body.
Pharmacodynamic effects. After partial conversion to liothyronine (T3), mainly in the liver and kidneys, and passage into body cells, the characteristic effects of thyroid hormones are observed on development, growth and metabolism.
Clinical efficacy and safety. Replacement of thyroid hormones leads to normalization of metabolic processes. For example, taking levothyroxine leads to a significant reduction in elevated cholesterol levels caused by hypothyroidism.
Pharmacokinetics
Suction. Absorption of orally administered levothyroxine occurs mainly in the upper part of the small intestine, the extent of absorption of which mainly depends on the galenic form of the drug and can be up to 80% when taken on an empty stomach. If the drug is taken with food, its absorption is significantly reduced. Cmax in blood plasma is achieved approximately 2–3 hours after administration. The therapeutic effect is noted on the 3rd–5th day after the start of oral administration.
Distribution. The volume of distribution is approximately 10–12 l. Levothyroxine binds to specific plasma proteins by approximately 99.97%. The binding of proteins to hormones is not covalent, so there is a constant and very rapid exchange between free and bound hormone.
Excretion. The metabolic clearance of levothyroxine is about 1.2 liters of blood plasma per day. Breakdown occurs mainly in the liver, kidneys, brain and muscles. Metabolites are excreted in urine and feces. T½ of the drug is about 7 days. With thyrotoxicosis, this period is reduced to 3–4 days, and with hyperthyroidism it is extended to 9–10 days.
During pregnancy and breastfeeding. Levothyroxine crosses the placenta only in small quantities. When taken in normal doses, levothyroxine passes into breast milk only in small quantities.
Renal dysfunction. Due to the high level of protein binding, neither hemodialysis nor hemoperfusion affects levothyroxine levels.
L-Thyroxine 100 µg TBL N100 /Germany/
L-Thyroxine 100 Berlin-Chemie - instructions for use
Trade name of the drug: L-Thyroxine 100 Berlin-Chemie
International nonproprietary name:
Levothyroxine sodium
Dosage form:
tablets
Composition:
Active ingredient: levothyroxine sodium - 0.100 mg.
Excipients: calcium hydrogen phosphate dihydrate - 31.90 mg, microcrystalline cellulose 32.00 mg, sodium carboxymethyl starch (type A) - 24.00 mg, dextrin - 13.60 mg, long-chain partial glycerides - 2.40 mg. Description: round, slightly convex tablets, white or off-white with a slightly yellowish tint, scored on one side and embossed 100 on the other.
Pharmacological group: Thyroid agent.
ATX code: H03AA01
Pharmacological properties Pharmacodynamics Synthetic levorotatory isomer of thyroxine.
After partial conversion into triiodothyronine (in the liver and kidneys) and passage into the cells of the body, it affects the development and growth of tissues and metabolism.
In small doses it has an anabolic effect on protein and fat metabolism. In medium doses, it stimulates growth and development, increases the need for oxygen in tissues, stimulates the metabolism of proteins, fats and carbohydrates, and increases the functional activity of the cardiovascular system and central nervous system. In large doses, it inhibits the production of thyrotropin-releasing hormone of the hypothalamus and thyroid-stimulating hormone (TSH) of the pituitary gland. The therapeutic effect is observed after 7-12 days, during the same time the effect persists after discontinuation of the drug. The clinical effect for hypothyroidism appears after 3-5 days. Diffuse goiter decreases or disappears within 3-6 months. Pharmacokinetics When taken orally, levothyroxine sodium is absorbed almost exclusively in the upper small intestine.
Up to 80% of the dose taken is absorbed.
Eating reduces the absorption of levothyroxine sodium. Maximum serum concentrations are achieved approximately 5-6 hours after oral administration. After absorption, more than 99% of the drug binds to serum proteins (thyroxine-binding globulin, thyroxine-binding prealbumin and albumin). Approximately 80% of sodium levothyroxine is monodeiodinated in various tissues to form triiodothyronine (T3) and inactive products. Thyroid hormones are metabolized mainly in the liver, kidneys, brain and muscles. A small amount of the drug undergoes deamination and decarboxylation, as well as conjugation with sulfuric and glucuronic acids (in the liver). Metabolites are excreted by the kidneys and through the intestines. The half-life of the drug is 6-7 days. With thyrotoxicosis, the half-life is shortened to 3-4 days, and with hypothyroidism it is extended to 9-10 days. Indications for use: hypothyroidism;
- euthyroid goiter; — as replacement therapy and for the prevention of goiter recurrence after resection of the thyroid gland; — thyroid cancer (after surgical treatment); - diffuse toxic goiter: after achieving a euthyroid state with antithyroid drugs (in the form of combination or monotherapy); - as a diagnostic tool when performing a thyroid suppression test. Contraindications - hypersensitivity to the active substance or to any of the excipients included in the drug (see.
section Composition);
- untreated thyrotoxicosis; - acute myocardial infarction, acute myocarditis; - untreated adrenal insufficiency. The drug should be prescribed with caution for diseases of the cardiovascular system: coronary artery disease (atherosclerosis, angina pectoris, history of myocardial infarction), arterial hypertension, arrhythmia, diabetes mellitus, severe long-term hypothyroidism, malabsorption syndrome (dose adjustment may be required).
Use during pregnancy and breastfeeding During pregnancy and breastfeeding, therapy with the drug prescribed for hypothyroidism should continue.
During pregnancy, an increase in the dose of the drug is required due to an increase in the content of thyroxine-binding globulin.
The amount of thyroid hormone secreted in breast milk during lactation (even when treated with high doses of the drug) is not enough to cause any problems in the child. The use of the drug in combination with antithyroid drugs during pregnancy is contraindicated, since taking levothyroxine sodium may require an increase in doses of antithyroid drugs. Since antithyroid drugs, unlike levothyroxine sodium, can cross the placenta, the fetus may develop hypothyroidism. During breastfeeding, the drug should be taken with caution, strictly in recommended doses under medical supervision. Method of administration and dosage The daily dose is determined individually depending on the indications.
L-Thyroxine 100 Berlin-Chemie in a daily dose is taken orally in the morning on an empty stomach, or at least 30 minutes before a meal, with a small amount of liquid (half a glass of water) and without chewing.
When carrying out replacement therapy for hypothyroidism (in the absence of cardiovascular diseases), L-Thyroxine 100 Berlin-Chemie is prescribed in a daily dose of 1.6-1.8 mcg/kg body weight. In case of significant obesity, the calculation should be made on the ideal weight. Initial stage of replacement therapy for hypothyroidism Patients without cardiovascular diseases under 55 years of age? initial dose: women - 50-100 mcg/day, men - 50-150 mcg/day Patients with cardiovascular diseases or over 55 years of age? The initial dose is 25 mcg per day? Increase by 25 mcg at intervals of 3-6 weeks until the TSH level in the blood normalizes? If symptoms from the cardiovascular system appear or worsen, correct the treatment of cardiovascular diseases. Infants and children under 3 years of age are given a daily dose of L-Thyroxine 100 Berlin-Chemie in one dose 30 minutes before the first feeding. The tablet is dissolved in water to a thin suspension, which is prepared immediately before taking the drug. In patients with severe long-term hypothyroidism, treatment should be started with extreme caution, with small doses - 25 mcg / day, the dose is increased to maintenance at longer intervals - by 25 mcg / day every 2 weeks and the TSH concentration in the blood is determined more often. For hypothyroidism, L-Thyroxine 100 Berlin-Chemie is usually taken throughout life. For thyrotoxicosis, L-Thyroxine 100 Berlin-Chemie is used in complex therapy with antithyroid drugs after achieving a euthyroid state. In all cases, the duration of treatment with the drug is determined by the doctor. Recommended doses of thyroxine for the treatment of congenital hypothyroidism Age Daily dose (mcg) Thyroxine dose per body weight (mcg/kg) 0-6 months 25-50 10-15 6-24 months 50-75 8-10 from 2 to 10 years 75-125 4-6 from 10 to 16 years 100-200 3-4 ] 16 years 100-200 2-3 Indications Recommended doses (L-Thyroxine 100 Berlin-Chemie, mcg/day) Treatment of euthyroid goiter 50-200 Prevention of relapse after surgical treatment of euthyroid goiter 50-200 In complex therapy of thyrotoxicosis 50-100 Suppressive therapy for thyroid cancer 150-300 Thyroid suppression test 4 weeks before the test
3 weeks before the test
2 weeks before the test
1 week before the test
L-Thyroxine 100 Berlin-Chemie 50 mcg/day 100 mcg/day 150-200 mcg/day 150-200 mcg/day For precise dosing of the drug, use the most suitable form of release of the drug L-Thyroxine Berlin-Chemie (50, 75, 100, 125 or 150 mcg).
Side effects When used correctly under medical supervision, no side effects are observed.
If you are hypersensitive to the drug, allergic reactions may occur.
Overdose In case of an overdose of the drug, symptoms characteristic of thyrotoxicosis are observed: tachycardia, cardiac arrhythmia, heart pain, anxiety, tremor, insomnia, hyperhidrosis, loss of appetite, weight loss, diarrhea, vomiting, headache, increased fatigue, muscle spasms.
Depending on the severity of symptoms, the doctor may recommend reducing the daily dose of the drug, a break in treatment for several days, or prescribing beta-blockers.
After side effects disappear, treatment should be started with caution at a lower dose. Antithyroid drugs are not recommended. Interaction with other drugs Levothyroxine sodium enhances the effect of indirect anticoagulants, which may require a reduction in their dose.
The use of tricyclic antidepressants with levothyroxine sodium may lead to increased effects of the antidepressants.
Thyroid hormones may increase the need for insulin and oral hypoglycemic agents. More frequent monitoring of blood glucose concentrations is recommended during the period of initiation of treatment with levothyroxine sodium, as well as when changing the dose of the drug. Levothyroxine sodium reduces the effect of cardiac glycosides. With simultaneous use of cholestyramine, colestipol and aluminum hydroxide, they reduce the plasma concentration of levothyroxine sodium by inhibiting its absorption in the intestine. When used simultaneously with anabolic steroids, asparaginase, tamoxifen, pharmacokinetic interaction is possible at the level of protein binding. When used simultaneously with phenytoin, salicylates, clofibrate, furosemide in high doses, the content of levothyroxine sodium and T4 not bound to blood plasma proteins increases. Somatotropin, when used simultaneously with levothyroxine sodium, can accelerate the closure of epiphyseal growth zones. Taking phenobarbital, carbamazepine and rifampicin may increase the clearance of levothyroxine sodium and require an increase in dose. Estrogens increase the concentration of the thyroglobulin-bound fraction, which may lead to a decrease in the effectiveness of the drug. Amiodarone, aminoglutethimide, para-aminosalicylic acid (PAS), ethionamide, antithyroid drugs, beta-blockers, chloral hydrate, diazepam, levodopa, dopamine, metoclopramide, lovastatin, somatostatin affect the synthesis, secretion, distribution and metabolism of levothyroxine sodium. Products containing soy may reduce the absorption of levothyroxine sodium (dose adjustment may be required). Special instructions In case of hypothyroidism caused by damage to the pituitary gland, it is necessary to find out whether there is simultaneous insufficiency of the adrenal cortex.
In this case, replacement therapy with glucocorticosteroids should be started before treatment of hypothyroidism with thyroid hormones is started in order to avoid the development of acute adrenal insufficiency.
The effect of the drug on the ability to drive vehicles and operate machinery L-Thyroxine 100 Berlin-Chemie does not affect the ability to drive vehicles and work requiring increased concentration. Release form Tablets 100 mcg.
25 tablets per blister pack (blister) [PVC/PVDC/aluminum foil or aluminum foil/aluminum foil].
1, 2 or 4 blisters along with instructions for use are placed in a cardboard box. Storage conditions Store at a temperature not exceeding 25 C.
Store the medicine out of the reach of children!
Shelf life: 2 years.
Do not use after the expiration date indicated on the package!
Conditions for dispensing from pharmacies By prescription.
The appearance of the product may differ from the photographs on the website.
Information about medications posted on the site is intended for specialists and includes materials from publications from different years. The information provided on the site is general and is presented for informational purposes only, does not replace consultation with a doctor and cannot serve as a guarantee of the positive effect of the drug. Tvoyaapteka.rf warns you about possible negative consequences that may arise as a result of incorrect use of the information presented on the site and strongly recommends that you consult with a specialist before choosing a drug.
You can find detailed and up-to-date instructions for the drug on the website of the State Register of Medicines www.grls.rosminzdrav.ru.
Indications
L-thyroxine 50 Berlin-Chemie, l-thyroxine 100 Berlin-Chemie:
- benign goiter with euthyroid state of thyroid function;
- prevention of goiter recurrence after goiter resection with a euthyroid state of thyroid function;
- replacement therapy for hypothyroidism of various etiologies;
- an adjuvant for thyreostatic therapy of hyperthyroidism after achieving a euthyroid functional state;
- suppressive and replacement therapy for thyroid cancer, mainly after thyroidectomy.
Additional indication for L-Thyroxine 100 Berlin-Chemie and L-Thyroxine 150 Berlin-Chemie:
- as a diagnostic tool when performing a thyroid suppression test.
L-Thyroxine 75 Berlin-Chemie, L-Thyroxine 125 Berlin-Chemie, L-Thyroxine 150 Berlin-Chemie:
- replacement therapy for hypothyroidism of various etiologies;
- prevention of goiter recurrence after goiter resection with a euthyroid state of thyroid function;
- benign goiter with euthyroid state of thyroid function;
- suppressive and replacement therapy for malignant tumors of the thyroid gland, mainly after thyroidectomy.
Additional indication for L-Thyroxine 75 Berlin-Chemie:
- an adjuvant for thyreostatic therapy of hyperthyroidism after achieving a euthyroid functional state.
Composition and release form
L-Thyroxine 50 Berlin-Chemie
| Pills | 1 table |
| levothyroxine sodium | 50 mcg |
| excipients: calcium hydrogen phosphate aqueous; MCC; carboxymethyl starch sodium salt (type A); dextrin; long-chain partial glycerides |
25 pcs in blister; There are 2 or 4 blisters in a cardboard pack.
L-Thyroxine 100 Berlin-Chemie
| Pills | 1 table |
| levothyroxine sodium | 100 mcg |
| excipients: calcium hydrogen phosphate aqueous; MCC; carboxymethyl starch sodium salt (type A); dextrin; long-chain partial glycerides |
25 pcs in blister; There are 2 or 4 blisters in a cardboard pack.
Application
L-thyroxine 50 Berlin-Chemie, l-thyroxine 100 Berlin-Chemie. Dosing data should be considered as recommendations. The individual daily dose is determined based on the results of laboratory tests and clinical examination. Thyroid hormone therapy should be started at a low dose and gradually increased (every 2–4 weeks) to the required therapeutic dose.
Because T4 or free thyroxine (fT4) levels may be elevated in some patients, plasma TSH concentrations are better suited to monitor treatment regimen.
Adult patients. Treatment of benign goiter: 75–200 mcg/day.
Prevention of goiter relapse: 75–200 mcg/day.
Replacement therapy for hypothyroidism: the initial dose is 25–50 mcg/day, maintenance dose is 100–200 mcg/day.
Concomitant therapy in the treatment of hyperthyroidism with thyreostatic drugs: 50–100 mcg/day.
Suppressive and replacement therapy for thyroid cancer: 150–300 mcg/day.
When performing a thyroid suppression test (for L-thyroxine 100 Berlin-Chemie): 200 mcg (2 tablets)/day (14 days before the test).
Children with congenital and acquired hypothyroidism. The maintenance dose is usually 100–150 mcg of levothyroxine per 1 m2 of body surface area per day.
For infants and children with congenital hypothyroidism who are candidates for immediate levothyroxine replacement therapy, the recommended starting dose of levothyroxine for the first 3 months is 10–15 mcg/kg/day. In the future, dose adjustment is carried out individually according to the results of clinical studies, taking into account the level of thyroid hormone, as well as the level of TSH.
For children with acquired hypothyroidism, the initial dose of levothyroxine is 12.5–50 mcg/day, for which the drug should be used in an appropriate dose. Based on clinical data regarding thyroid hormone levels, as well as TSH levels, the dose should be increased gradually at intervals of 2-4 weeks until the full dose required for replacement therapy is reached. For infants and children under 3 years of age, the full daily dose is added at least 30 minutes before the first feeding of the day. The tablets are pre-dissolved in a small amount of water (10–15 ml), and the resulting freshly prepared suspension is given to the child, adding a small amount of water (5–10 ml).
Elderly patients. In some cases, in elderly people, for example in patients with heart disease, preference should be given to a gradual reduction in the dose of levothyroxine sodium with constant determination of TSH levels.
Experience shows that using the minimum dose is the optimal solution for low body weight and large nodular goiter.
The entire daily dose should be swallowed without chewing the tablets, washed down with a small amount of liquid, for example ½ glass of water. Take the drug on an empty stomach, at least 30 minutes before breakfast.
Thanks to the special shape of the tablet, it can be divided as follows: place the tablet on a hard surface with the division notch facing up and press it with your finger from above in a perpendicular direction.
Duration of treatment. The drug is used throughout life for hypothyroidism, after surgical interventions - strumectomy or thyroidectomy, as well as to prevent relapses after removal of euthyroid goiter. The duration of use of the drug as an adjuvant for the treatment of hyperthyroidism after achieving a euthyroid functional state corresponds to the duration of thyreostatic therapy. For mild forms of euthyroid goiter, the duration of treatment ranges from 6 months to 2 years. If the patient's condition does not improve after treatment, then surgery or radioactive iodine therapy is prescribed.
Thyroid suppression test. When performing a thyroid suppression test, take 150–200 mcg of levothyroxine sodium daily for 14 days.
L-Thyroxine 75 Berlin-Chemie, L-Thyroxine 125 Berlin-Chemie, L-Thyroxine 150 Berlin-Chemie. Dosing data should be considered as guidelines. The individual daily dose is determined based on the results of laboratory tests and clinical examination. If minimal thyroid function is maintained, the minimum effective dose should be used.
In elderly patients, patients with coronary artery disease and with severe or chronic hypothyroidism, treatment with thyroid hormones should be initiated with extreme caution, for example, it is recommended to start treatment with a low dose and increase it slowly, at long intervals, frequently monitoring the level of thyroid hormones. Considering the experience of use both in patients with low body weight and in patients with large nodular goiter, lower doses of the drug are sufficient.
Because T4 or free thyroxine (fT4) levels may be elevated in some patients, serum TSH concentrations are better suited to monitor treatment regimen.
Adult patients
Hypothyroidism. The initial dose is 25–50 mcg/day, maintenance dose is 100–200 mcg/day (the dose is increased by 25–50 mcg at intervals of 2–4 weeks).
Prevention of goiter relapse - 75–200 mcg/day.
Benign goiter with a euthyroid state of function - 75–200 mcg/day.
Concomitant therapy in the treatment of hyperthyroidism with thyreostatics - 50–100 mcg/day.
After thyroidectomy for a malignant tumor - 150–300 mcg/day.
When performing a thyroid suppression test (for L-thyroxine 150 Berlin-Chemie): 150 mcg (1 tablet)/day (14 days before the test).
If dose adjustment of this drug is not possible, there are medications available in other dosages. You should consult your doctor for advice.
Children with congenital and acquired hypothyroidism. The maintenance dose is usually 100–150 mcg of levothyroxine per 1 m2 of body surface area per day.
For infants and children with congenital hypothyroidism who are candidates for immediate levothyroxine replacement therapy, the recommended starting dose of levothyroxine for the first 3 months is 10–15 mcg/kg/day. In the future, dose adjustment is carried out individually according to the results of clinical studies, taking into account the level of thyroid hormone, as well as the level of TSH.
For children with acquired hypothyroidism, the initial dose of levothyroxine is 12.5–50 mcg/day, for which the drug should be used in an appropriate dose. Based on clinical data regarding thyroid hormone levels, as well as TSH levels, the dose should be increased gradually at intervals of 2-4 weeks until the full dose required for replacement therapy is reached. For children, take the full daily dose at least 30 minutes before the first feeding of the day. The tablets can also be taken in suspension form. The tablets are pre-dissolved in a small amount of water (10–15 ml), and the resulting freshly prepared suspension is given to the child, adding a small amount of water (5–10 ml).
Elderly patients. In some cases, in elderly patients, for example in patients with heart disease, preference should be given to a gradual reduction in the dose of levothyroxine sodium with constant determination of TSH levels.
The entire daily dose should be swallowed without chewing the tablets and washed down with a small amount of liquid. Take the drug on an empty stomach, at least 30 minutes before breakfast.
Thanks to the special shape of the tablet, it can be divided as follows: place the tablet on a hard surface with the division notch facing up and press it with your finger from above in a perpendicular direction.
Duration of treatment
The drug is usually used throughout life for hypothyroidism and after thyroidectomy due to a malignant tumor of the thyroid gland; for euthyroid goiter and for the prevention of goiter relapses - from several months or years to lifelong use, as an adjuvant in the treatment of hyperthyroidism - depending on the duration of thyreostatic therapy.
The duration of treatment for euthyroid goiter should be from 6 months to 2 years. If the patient's condition does not improve after treatment with L-thyroxine 75 Berlin-Chemie or L-thyroxine 125 Berlin-Chemie or L-thyroxine 150 Berlin-Chemie, other therapeutic approaches should be considered.
Thyroid suppression test. When performing a thyroid suppression test, take 150–200 mcg of levothyroxine sodium daily for 14 days.
Indications of the drug L-Thyroxine 100 Berlin-Chemie
Hypothyroidism (underfunction of the thyroid gland) of any origin: primary and secondary hypothyroidism, after operations for struma, as a result of therapy with radioactive iodine (as replacement therapy).
Prevention of relapse (re-formation) of nodular goiter after surgery for goiter with normal thyroid function.
Diffuse goiter with normal function.
As part of combination therapy in the treatment of hyperfunction of the thyroid gland with thyreostatics after achieving its normal function.
Malignant tumor of the thyroid gland, mainly after surgery to suppress tumor recurrence and as replacement therapy.
Contraindications
Hypersensitivity to the active substance or any of the components of the drug; untreated hyperthyroidism of any etiology; untreated adrenal insufficiency; untreated pituitary insufficiency; acute myocardial infarction; acute myocarditis; acute pancarditis.
During pregnancy, the simultaneous use of levothyroxine and any thyreostatic agent is contraindicated. (For more detailed information on use during pregnancy and lactation, see section Use during pregnancy and lactation).
Side effects
If the patient does not tolerate the dose of the drug, which is very rare, or in case of overdose, especially if the dose is increased too quickly at the beginning of treatment, typical symptoms of hyperthyroidism may occur.
In these cases, it is recommended to reduce the daily dose or stop using the drug for several days. When side effects disappear, treatment is resumed, carefully selecting the dose of the drug.
If you are hypersensitive to levothyroxine or any of the excipients of the drug, allergic reactions from the skin (for example, skin rash, urticaria) and respiratory tract may occur. There are isolated reports of the development of anaphylactic shock. In this case, use of the drug should be discontinued.
Adverse reactions are classified by frequency of occurrence as follows: very often (≥1/10); often (from ≥1/100 to 1/10); sometimes (from ≥1/1000 to 1/100); rare (≥1/10,000 to 1/1000); very rare (1/10,000); frequency unknown (cannot be estimated from available data).
From the immune system: frequency unknown - hypersensitivity.
From the side of the heart: very often - rapid heartbeat; often - tachycardia; unknown - arrhythmia, angina pectoris.
From the skin and subcutaneous tissue: frequency unknown - rash, urticaria, hyperhidrosis.
Mental disorders: very often - insomnia; often - nervousness; frequency unknown - feeling of inner restlessness.
From the musculoskeletal system and connective tissue: frequency unknown - muscle weakness, muscle cramps, osteoporosis during the use of depressing doses of levothyroxine, especially in postmenopausal women, mainly during long-term treatment.
Vascular: frequency unknown - sensation of heat, collapse (acute vascular insufficiency) in premature infants with very low birth weight (see SPECIAL INSTRUCTIONS).
From the reproductive system and mammary gland: frequency unknown - menstrual irregularities.
From the gastrointestinal tract: frequency unknown - diarrhea, vomiting.
Results of additional research methods: frequency unknown - decrease in body weight.
From the nervous system: very often - headache; rarely - pseudotumor cerebri (mainly in children); frequency unknown - tremor.
General disorders and reactions at the injection site: frequency unknown - heat intolerance, fever.
Reporting suspected adverse reactions. It is extremely important to report suspected adverse reactions after drug registration. This makes it possible to continuously monitor the benefit/risk ratio of the drug. Health professionals are asked to ensure that any suspected adverse reactions are reported through the national reporting system.
Side effects
Allergic reactions (skin rash, itchy skin). When used in excessively high doses - hyperthyroidism (changes in appetite, dysmenorrhea, chest pain, diarrhea, tachycardia, arrhythmia, fever, tremor, headache, irritability, muscle cramps of the lower extremities, nervousness, sweating, difficulty falling asleep, vomiting, weight loss body). When used in insufficiently effective doses - hypothyroidism (dysmenorrhea, constipation, dryness, puffiness of the skin, headache, lethargy, myalgia, drowsiness, weakness, apathy, weight gain).
special instructions
Before starting thyroid hormone therapy or thyroid suppression tests, it is necessary to exclude the presence or treat the following diseases or conditions: ischemic heart disease, angina pectoris, hypertension, pituitary insufficiency and/or adrenal insufficiency.
Before starting treatment with thyroid hormones, the functional autonomy of the thyroid gland should also be excluded or treated.
In the case of coronary artery disease, heart failure, tachyarrhythmia, myocarditis in remission, chronic hypothyroidism or in patients who have suffered a myocardial infarction, it is imperative to avoid pharmacologically induced hyperthyroidism, even mild degrees.
When conducting thyroid hormone therapy in these patients, thyroid hormone levels should be monitored frequently (see APPLICATION).
In the case of secondary hypothyroidism, it is necessary to check for concomitant adrenal insufficiency. When this disease is detected, replacement therapy (hydrocortisone) should be performed first. Without adequate corticosteroid supply, thyroid hormone therapy in patients with adrenal insufficiency or pituitary insufficiency can lead to Addisonian crisis.
Because of immature adrenal function and possible collapse (acute vascular insufficiency) (see ADVERSE EFFECTS), extreme caution should be used when initiating levothyroxine therapy in very low birth weight preterm infants.
If you suspect the presence of autonomous thyroiditis, you should determine the TSH level or perform thyroid scintigraphy before starting treatment.
In postmenopausal women, there is an increased risk of developing osteoporosis, therefore, it is necessary to titrate the dose of levothyroxine to achieve the minimum effective dose, and also, in order to avoid increasing the concentration of levothyroxine in the blood above the physiological level, thyroid function should be checked in these patients more often (see . SIDE EFFECTS).
Thyroid hormones should not be used to reduce body weight. The administration of physiological doses does not lead to a decrease in body weight in patients with a euthyroid state. Higher doses may cause serious or even life-threatening adverse reactions, especially when combined with certain weight loss medications.
If a levothyroxine therapy regimen is established, switching to another drug containing thyroid hormones should only be carried out under the supervision of laboratory tests and clinical data.
In patients concomitantly taking levothyroxine and other drugs that may affect the thyroid gland (amiodarone, tyrosine kinase inhibitors, salicylates and furosemide in high doses), monitoring of thyroid function is necessary (see INTERACTIONS).
For patients with diabetes mellitus and patients receiving anticoagulants, see INTERACTIONS.
Cases of hypothyroidism have been reported in patients receiving sevelamer and levothyroxine concomitantly. Therefore, TSH levels should be carefully monitored in such patients receiving both drugs (also see INTERACTIONS).
Use during pregnancy and lactation
Pregnancy. Treatment with thyroid hormones must be carried out consistently during pregnancy.
To ensure optimal maternal and fetal health, it is important that thyroid hormone levels remain within normal limits.
Despite its widespread use during pregnancy, there is no evidence of side effects of levothyroxine on pregnancy or the health of the fetus/newborn.
Due to estrogens, the need for levothyroxine may increase during pregnancy. For this reason, during pregnancy, thyroid function should be monitored and, if necessary, the dose of thyroid hormone should be adjusted.
During pregnancy, the use of levothyroxine sodium as an adjuvant in the treatment of hyperthyroidism with thyreostatic drugs is contraindicated. Additional intake of levothyroxine may require an increase in the dose of thyreostatics. Thyrostatic drugs, unlike levothyroxine, penetrate the placental barrier in significant doses. This can lead to the development of fetal hypothyroidism. For this reason, in pregnant women with hyperthyroidism, thyreostatics should always be used as monotherapy and in low doses.
During pregnancy, it is prohibited to perform a thyroid suppression test.
Lactation. Treatment with thyroid hormones must be carried out consistently during breastfeeding. Currently, there is no information about the side effects of levothyroxine on the health of the newborn. The amount of thyroid hormone that passes into breast milk during breastfeeding, even with high-dose thyroid hormone therapy, is insufficient to cause infants to develop hyperthyroidism or suppress TSH secretion.
Estrogen may increase the need for levothyroxine during pregnancy. Therefore, after pregnancy, thyroid function should be monitored and, if necessary, the dose of thyroid hormone should be adjusted.
During breastfeeding, it is prohibited to perform a thyroid suppression test.
Children. The drug is used in pediatric practice. Detailed information on recommended doses and methods of use of the drug is given in the APPLICATION section.
The ability to influence reaction speed when driving vehicles or working with other mechanisms. No relevant studies have been conducted.
pharmachologic effect
Pharmacological action - replenishes the deficiency of thyroid hormones, thyroid.
After partial conversion into liothyronine (in the liver and kidneys) and passage into the cells of the body, it affects the development and growth of tissues and metabolism. In small doses it has an anabolic effect on protein and fat metabolism. In medium doses, it stimulates growth and development, increases tissue oxygen demand, stimulates the metabolism of proteins, fats and carbohydrates, and increases the functional activity of the cardiovascular system and central nervous system. In large doses, it inhibits the production of thyrotropin-releasing hormone of the hypothalamus and thyroid-stimulating hormone of the pituitary gland.
Interactions
Antidiabetic drugs. Levothyroxine may reduce the hypoglycemic effect of antidiabetic drugs in the blood (for example, metformin, glimepiride, glibenclamide and insulin). It is recommended to frequently monitor blood glucose levels in diabetes mellitus, especially at the beginning and end of treatment with thyroid hormones, and, if necessary, adjust the dose of the glucose-lowering drug.
Coumarin derivatives Levothyroxine can enhance the effect of coumarin derivatives by displacing them from sites of binding to plasma proteins. Therefore, in case of simultaneous use, it is necessary to regularly monitor coagulation parameters, and, if necessary, adjust (reduce) the dose of anticoagulants.
Ion exchange resins. Ion exchange resins, such as cholestyramine, colestipol, or the calcium and sodium salts of polystyrene sulfonic acid, inhibit the absorption of levothyroxine by binding thyroid hormones in the gastrointestinal tract; therefore, they should not be used earlier than 4–5 hours after taking L-Thyroxine Berlin Hemie.
Bile acid binders. Colesevelam binds to levothyroxine and thus reduces the absorption of levothyroxine from the gastrointestinal tract. No interaction was observed when levothyroxine was administered at least 4 hours before colesevelam. Therefore, L-Thyroxine Berlin-Chemie should be used at least 4 hours before taking colesevelam.
Aluminum-containing antacid preparations, as well as iron- and calcium-containing preparations. The absorption of levothyroxine may be reduced in case of simultaneous use of aluminum-containing antacids (antacids, sucralfate), iron-containing drugs and calcium-containing drugs. Therefore, L-Thyroxine Berlin-Chemie should be taken at least 2 hours before taking these medications.
Sevelamer and lanthanum carbonate. Sevelamer and lanthanum carbonate may reduce the bioavailability of levothyroxine (also see WARNINGS).
Propylthiouracil, corticosteroids and beta-adrenergic blockers (especially propranolol). These substances inhibit the conversion of thyroxine (T4) to T3 and can lead to a decrease in the concentration of T3 in the blood plasma.
Amiodarone and iodinated radiocontrast agents. Due to their high iodine content, amiodarone and iodinated contrast agents can cause both hyper- and hypothyroidism. Particular care should be taken in case of nodular goiter with possible uncertain autonomy. Amiodarone inhibits the conversion of T4 to T3, resulting in a decrease in the concentration of T3 and an increase in the level of TSH in the blood plasma. Due to the effect of amiodarone on thyroid function, it may be necessary to adjust the dose of L-Thyroxine Berlin-Chemie.
Salicylates, dicumarol, furosemide, clofibrate. Salicylates (especially at a dose of 2 g/day), dicumarol, furosemide in high doses (250 mg), clofibrate and other substances can displace levothyroxine from binding sites with blood plasma proteins. This may lead to an initial transient increase in free thyroid hormone levels, which leads to a decrease in total thyroid hormone levels.
Estrogen-containing contraceptives, drugs for hormone replacement therapy during the postmenopausal period. The need for levothyroxine may increase during the use of estrogen-containing contraceptives or hormone replacement therapy in the postmenopausal period. Increased binding of levothyroxine is possible, which can lead to diagnostic and treatment errors.
Sertraline, chloroquine/proguanil These substances reduce the effectiveness of levothyroxine and increase serum TSH levels.
Enzyme-inducing drugs. Barbiturates, rifampicin, carbamazepine, phenytoin and other drugs that can activate liver enzymes may increase the hepatic clearance of levothyroxine and lead to a decrease in its plasma levels.
Protease inhibitors (eg ritonavir, indinavir, lopinavir). There are reports of loss of the therapeutic effect of levothyroxine when combined with lopinavir/ritonavir. Therefore, in patients taking levothyroxine and protease inhibitors concomitantly, careful monitoring of clinical symptoms and thyroid function is necessary. If necessary, the dose of levothyroxine should be adjusted.
Tyrosine kinase inhibitors. Tyrosine kinase inhibitors (eg imatinib, sunitinib, sorafenib, motesanib) may reduce the effectiveness of levothyroxine. Therefore, in patients taking levothyroxine and tyrosine kinase inhibitors concomitantly, careful monitoring of clinical symptoms and thyroid function parameters is necessary. If necessary, the dose of levothyroxine should be adjusted.
Preparations containing soy. Preparations containing soy may inhibit the absorption of levothyroxine in the intestine.
Elevated serum TSH levels have been reported in children on a soy diet and treated with levothyroxine due to congenital hypothyroidism. To achieve normal serum T4 and TSH levels, high doses of levothyroxine are recommended. During and after completion of the soy diet, careful monitoring of serum T4 and TSH levels is necessary, and it may be necessary to adjust the dose of levothyroxine.
Pharmacokinetics
When taken orally, levothyroxine is absorbed almost exclusively in the upper small intestine. Up to 80% of the dose taken is absorbed. Eating reduces the absorption of levothyroxine. Cmax in blood serum is achieved 6 hours after administration. After absorption, more than 99% of the drug is bound to serum proteins. In various tissues, monodeiodination of levothyroxine occurs to form triiodothyronine and inactive products. Thyroid hormones are metabolized mainly in the liver, kidneys, brain and muscles. A small amount of the drug undergoes deamination and decarboxylation, as well as conjugation with sulfuric and glucuronic acids (in the liver). Metabolites are excreted in urine and bile. T1/2 - 6–8 days.
Overdose
In case of overdose, rapid pulse, increased heart rate, anxiety, feeling of heat, increased body temperature, increased sweating, arrhythmia, insomnia, tremor, increased frequency of angina attacks, anxiety, weight loss, vomiting, diarrhea, headache, weakness and muscle cramps are observed. , menstrual irregularities, pseudotumor cerebri. There have been reports of isolated cases of epileptic seizures in the corresponding category of patients when the individual tolerable dose limit was exceeded. It is recommended to stop taking the drug and conduct a follow-up examination.
An increase in T3 levels is a more reliable indicator of drug overdose than an increase in T4 and fT4 levels.
In case of overdose and intoxication, symptoms characteristic of a moderate or significant acceleration of metabolism occur (see SIDE EFFECTS). Depending on the degree of overdose, it is recommended to stop taking the drug and conduct a follow-up examination.
In cases of intoxication in humans (attempted suicide), levothyroxine in a dose of up to 10 mg is tolerated without complications. The development of such serious complications as disruption of vital functions (respiration and circulation) is unlikely if there is no history of coronary artery disease. Despite this, thyrotoxic crisis, seizures, heart failure and coma have been reported. There have been isolated reports of cases of sudden death associated with cardiac dysfunction in patients taking high doses of levothyroxine for a long time.
In cases of acute overdose, absorption of the drug in the gastrointestinal tract can be reduced by taking activated charcoal. Treatment is usually symptomatic and supportive. Severe beta-sympathomimetic symptoms, such as tachycardia, anxiety, agitation, or hyperkinesia, can be reduced by the use of beta-blockers. The use of thyreostatics is not indicated, since the function of the thyroid gland is already completely suppressed.
In case of taking the drug in extremely high doses (suicide attempt), it is advisable to perform plasmapheresis.
In case of overdose of levothyroxine, long-term observation is necessary. Due to the gradual conversion of levothyroxine to liothyronine, the development of symptoms may be delayed by up to 6 days.
Note!
Description of the drug L-thyroxine 100 Berlin-Chemie tablet. 100mcg No. 50 on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.