Maltofer Fol, 30 pcs., 100 mg+0.35 mg, chewable tablets
Suction.
Iron from iron(III) hydroxide polymaltose is absorbed according to a controlled mechanism.
The increase in serum iron after drug administration does not correlate with total iron absorption measured as incorporation into hemoglobin ( Hb
).
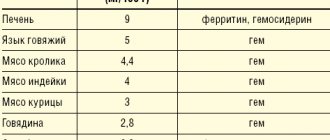
Studies with radiolabeled iron(III) hydroxide polymaltose have shown a strong correlation between iron incorporation into red blood cells and iron content throughout the body. The maximum activity of absorption of iron from iron (III) hydroxide polymaltose is observed in the duodenum and small intestine. As with other oral iron supplements, the relative absorption of iron from iron(III) polymaltose hydroxide, measured as incorporation into Hb
, decreases with increasing iron doses. In addition, a correlation was observed between the severity of iron deficiency (in particular serum ferritin concentration) and the relative amount of iron absorbed (i.e., the more severe the iron deficiency, the better the relative absorption). In anemic patients, iron absorption from iron(III) polymaltose hydroxide, in contrast to iron salts, was increased in the presence of food.
Folic acid is absorbed primarily in the gastrointestinal tract, in particular in the duodenum and small intestine. When taking folic acid at a dose of 0.35 mg, absorption is about 80%.
Distribution.
The distribution of iron from iron(III) hydroxide polymaltose after absorption was studied in a study using the dual isotope technique (55Fe and 59Fe).
Cmax of folic acid in the blood is achieved in 30–60 minutes. In a single-dose study in 12 healthy women, folic acid from Maltofer® Fol, chewable tablets (100 mg iron, 0.35 mg folic acid) was shown to be rapidly absorbed, with a plasma folate Cmax of 11 ng/ml , is achieved 0.75 hours after taking the drug. Folic acid intensively binds to blood plasma proteins, penetrates the blood-brain barrier, the placenta and into breast milk.
Biotransformation.
Absorbed iron binds to transferrin and is used for the synthesis
of Hb
in the bone marrow or is stored mainly in the liver, where it binds to ferritin.
Folic acid is metabolized in the cells of the small intestine and liver, as well as in other organs. After this, folates bound to transport proteins are distributed to all organs.
Excretion.
Unabsorbed iron is excreted in the intestines (with feces).
Excretion of folic acid occurs mainly by the kidneys, as well as through the digestive tract. Folic acid is removed by hemodialysis.
Maltofer Fol chewable tablets No. 30
Compound
Active ingredients: iron (III) hydroxide polymaltosate - 357 mg, equivalent to iron content - 100 mg; folic acid - 0.35 mg. Excipients: dextrates - 232 mg, macrogol 6000 - 37 mg, purified talc - 21 mg, sodium cyclamate - 9 mg, vanillin - 2.9 mg, cocoa powder - 29 mg, chocolate flavor - 0.6 mg, microcrystalline cellulose - up to 730 mg.
Pharmacokinetics
Suction
Iron from iron(III) hydroxide polymaltose is absorbed according to a controlled mechanism. The increase in serum iron after dosing does not correlate with total iron absorption measured as incorporation into hemoglobin. Studies with radiolabeled iron(III) polymaltosate hydroxide have revealed a strong correlation between iron incorporation into red blood cells and iron content throughout the body. The maximum activity of absorption of iron from iron (III) hydroxide polymaltose is observed in the duodenum and small intestine. As with other oral iron preparations, the relative absorption of iron from iron(III) polymaltose hydroxide, measured as incorporation into hemoglobin, decreases with increasing doses of iron. In addition, a correlation was observed between the severity of iron deficiency (in particular, serum ferritin concentration) and the relative amount of iron absorbed (i.e., the more severe the iron deficiency, the better the relative absorption). In anemic patients, iron absorption from iron(III) polymaltose hydroxide, in contrast to iron salts, was increased in the presence of food.
Folic acid is absorbed from the gastrointestinal tract mainly in the duodenum and small intestine. When taking folic acid at a dose of 0.35 mg, absorption is about 80%.
Distribution
Iron absorbed from the gastrointestinal tract is transferred to the blood, where it immediately binds to transferrin. Iron bound to transferrin is distributed to depot tissues and organs, such as the liver and spleen. The distribution of iron from iron(III) hydroxide polymaltose after absorption was studied in a study using the dual isotope technique (55Fe and 59Fe).
Cmax of folic acid in the blood is achieved after 30-60 minutes. In a single-dose study in 12 healthy women, it was shown that folic acid from Maltofer® Fol, chewable tablets (100 mg iron, 0.35 mg folic acid) was rapidly absorbed, with a plasma folate Cmax of 11 ng/ml achieved after 0.75 hours after taking the drug. Folic acid intensively binds to blood plasma proteins, penetrates the BBB, the placental barrier and is excreted in breast milk.
Metabolism
Absorbed iron binds to transferrin and is used for the synthesis of hemoglobin in the bone marrow or is stored mainly in the liver, where it binds to ferritin.
Most iron is incorporated into the oxygen transport protein hemoglobin during erythropoiesis in the bone marrow or stored as ferritin. Iron from red blood cells is utilized after their breakdown in the spleen.
The breakdown products of polymaltose (maltose and gluconate) are converted into glucose, which is used in intermediate metabolism.
Folic acid is metabolized in the cells of the small intestine and liver, as well as in other organs. After this, folates bound to transport proteins are distributed to all organs.
Removal
Unabsorbed iron is excreted in the feces.
Excretion of folic acid occurs mainly by the kidneys, as well as through the digestive tract. Folic acid is removed by hemodialysis.
Indications for use
- treatment and prevention of iron deficiency without anemia (latent iron deficiency) and clinically significant iron deficiency anemia;
- treatment and prevention of iron and folic acid deficiency during pregnancy and breastfeeding.
Contraindications
- known hypersensitivity to iron (III) hydroxide polymaltose, folic acid or any excipient;
- iron overload (hemochromatosis, hemosiderosis);
- impaired iron utilization (lead anemia, sideroachrestic anemia, thalassemia);
- anemia not associated with iron deficiency (hemolytic anemia or megaloblastic anemia caused by vitamin B12 deficiency);
- children under 12 years of age (due to the need to prescribe small doses in this age group, it is recommended to use the drug Maltofer®, oral drops 50 mg/ml or Maltofer®, syrup 10 mg/ml).
Directions for use and doses
For oral administration.
The daily dose of the drug can be divided into several doses or taken at a time.
Maltofer® Fol should be taken during or immediately after a meal; the tablets can be chewed or swallowed whole.
Treatment of iron deficiency anemia outside of pregnancy
1 tablet 1-3 times a day for 3-5 months until normal hemoglobin (Hb) levels are achieved. After this, taking the drug should be continued at 1 tablet per day for several more months in order to restore iron reserves in the body.
Treatment of iron deficiency anemia during pregnancy
2 to 3 tablets (from 200 mg iron and 0.70 mg folic acid to 300 mg iron and 1.05 mg folic acid) per day until normal hemoglobin (Hb) levels are achieved. After this, treatment should be continued at least until the end of pregnancy, taking 1 tablet (100 mg of iron and 0.35 mg of folic acid) per day, in order to replenish iron stores and meet the increased need for iron due to pregnancy.
Maltofer® Fol is not recommended for use in children under 12 years of age.
Storage conditions
In a place protected from light at a temperature not exceeding 25°C.
Keep out of the reach of children.
Best before date
5 years. Do not use after the expiration date stated on the package.
special instructions
It is assumed that taking the drug Maltofer® Fol should not affect the daily need for insulin in patients with diabetes mellitus. 1 chewable tablet contains 0.04 XE.
The drug Maltofer® Fol contains folic acid, the intake of which can lead to masking of vitamin B12 deficiency.
Infectious diseases or malignancies can cause anemia. Since iron can be taken only after the underlying cause of the disease has been eliminated, the benefit-risk ratio of treatment should be determined.
During treatment with Maltofer® Fol, dark coloration of the stool may be observed, but this has no clinical significance.
Description
Antianemic drug.
Dosage form
Chewable tablets, brown with white inclusions, round, flat-cylindrical, scored.
Use in children
The use of the drug is contraindicated for children under 12 years of age.
Pharmacodynamics
In iron(III) hydroxide polymaltosate, polynuclear iron(III) hydroxide is externally surrounded by multiple covalently linked polymaltosate molecules, resulting in an overall average molecular weight of approximately 50 kDa. The structure of the active substance of the drug Maltofer® Fol is similar to the structure of the core protein ferritin - a physiological iron depot. Iron (III) hydroxide polymaltosate is stable and does not release large amounts of iron ions under physiological conditions. Due to its size, the degree of diffusion of iron(III) hydroxide polymaltosate through the mucosa is approximately 40 times less compared to iron(II) hexahydrate complex. Iron from iron (III) hydroxide polymaltose is actively absorbed in the intestine.
Folic acid (folate) belongs to the group of B vitamins. It is a precursor of tetrahydrofolate, which is a coenzyme of various metabolic processes, incl. biosynthesis of purines and nucleic acid thymidylates; it is necessary for the synthesis of nucleoproteins and for the maintenance of normal erythropoiesis.
The effectiveness of Maltofer® Fol for normalizing hemoglobin levels and replenishing iron depots has been demonstrated in numerous randomized controlled clinical trials using placebo control or an active comparator drug, conducted in adults and children with different iron depot status.
Side effects
The adverse reactions presented below are listed according to the damage to organs and organ systems and the frequency of occurrence. The frequency of occurrence is defined as follows: very common (≥1/10), common (≥1/100 and <1/10), uncommon (≥1/1000 and <1/100), rare (≥1/10000 and <1 /1000), very rare (<1/10000, including isolated cases).
The following adverse reactions were reported after using the drug Maltofer® during clinical trials, as well as in post-marketing studies:
From the nervous system: infrequently - headache.
From the digestive system: very often - change in the color of stool1; often - diarrhea, nausea, abdominal pain2, constipation; infrequently - vomiting3, discoloration of tooth enamel, gastritis.
From the skin and subcutaneous tissues: infrequently - itching, rash5,6, urticaria6, erythema6.
From the musculoskeletal system: rarely - muscle spasms4, myalgia.
Spontaneous post-marketing reports of adverse drug reactions
No additional adverse reactions were noted.
Deviations in laboratory parameters
No data available.
1 Changes in stool color were noted with less frequency in the meta-analysis, but are a well-studied reaction that occurs with oral iron treatment in general. In this regard, this adverse reaction was assigned a frequency of occurrence of “very common”.
2Includes: abdominal pain, dyspepsia, epigastric discomfort, bloating.
3Includes: vomiting, belching.
4Includes: involuntary muscle contractions, tremors.
5Includes: rash, macular rash, vesicular rash.
6Adverse reactions that were observed in the post-marketing period with an estimated incidence of <1/491 patients (upper limit of the 95% confidence interval).
Use during pregnancy and breastfeeding
Pregnancy
There are no data from clinical studies on the use of Maltofer® Fol in the first trimester of pregnancy. To date, there have been no reports of serious adverse reactions after taking Maltofer® Fol orally in therapeutic doses for the treatment of anemia during pregnancy. Data obtained from animal studies did not show danger to the fetus and mother.
In studies conducted in pregnant women after the end of the first trimester of pregnancy, no undesirable effects of the drug Maltofer® Fol were found in relation to mothers and/or newborns. In this regard, an adverse effect on the fetus when using the drug Maltofer® Fol is unlikely.
Breastfeeding period
A woman's breast milk contains iron bound to lactoferrin. The amount of iron transferred from iron(III) hydroxide polymaltose into breast milk is unknown. It is unlikely that the use of Maltofer® Fol by women who are breastfeeding can lead to undesirable effects in the child.
As a precautionary measure, women of childbearing age, women during pregnancy and during breastfeeding should take Maltofer® Fol only after consulting a doctor and in accordance with the “Dosage regimen” section. A benefit-risk assessment is recommended.
Interaction
The interaction of iron (III) hydroxide polymaltosate with tetracycline and aluminum hydroxide was studied. There was no significant decrease in tetracycline absorption. The concentration of tetracycline in the blood plasma did not fall below the minimum inhibitory concentration required for bacteriostasis. The absorption of iron from iron(III) hydroxide polymaltose was not reduced by aluminum hydroxide or tetracycline. Thus, iron (III) hydroxide polymaltosate can be used simultaneously with tetracycline and other phenolic compounds, as well as aluminum hydroxide.
In studies in rats using tetracycline, aluminum hydroxide, acetylsalicylic acid, sulfasalazine, calcium carbonate, calcium acetate and calcium phosphate in combination with vitamin D3, bromazepam, magnesium aspartate, D-penicillamine, methyldopa, paracetamol and auranofin, no interaction with iron was found (III) polymaltose hydroxide.
There is also no interaction of iron (III) polymaltosate hydroxide with food components such as phytic acid, oxalic acid, tannin, sodium alginate, choline and choline salts, vitamin A, vitamin D3 and vitamin E, soybean oil and soybean flour. These results suggest that iron(III) hydroxide polymaltosate can be taken during or immediately after meals.
Taking the drug does not affect the results of determining occult blood in feces (with selective determination of hemoglobin), so there is no need to interrupt treatment.
The simultaneous use of iron preparations for parenteral administration and oral administration should be avoided, since the absorption of iron taken orally is slowed down.
Treatment with folic acid may increase the metabolism of phenytoin, resulting in decreased serum phenytoin concentrations, especially in patients with folate deficiency. Although this interaction is not clinically significant, some patients may experience an increase in the frequency of seizures. Patients receiving phenytoin or other anticonvulsants should consult their physician before taking a product containing folic acid.
It has been found that the simultaneous use of chloramphenicol and folic acid in patients with folate deficiency may lead to a weakening of the hematopoietic response to folic acid due to the antagonistic effect of chloramphenicol. Although the significance and mechanism of the interaction are unclear, patients receiving both drugs concomitantly should be closely monitored for hematologic response to folic acid treatment.
Overdose
In case of overdose of Maltofer® Fol, iron overload or intoxication is unlikely, which is due to the low toxicity of iron (III) hydroxide polymaltosate and controlled iron uptake. No cases of unintentional fatal poisoning have been reported.
There are reports that excessive doses of folic acid can cause central nervous system disorders (in particular, changes in mental status, sleep disturbance, irritability and hyperactivity), nausea, bloating and flatulence.
Impact on the ability to drive vehicles and operate machinery
There are no data on the effect on the ability to drive vehicles or operate other machinery. However, some undesirable reactions (such as tremors, involuntary muscle contractions, etc. (see section “Side Effects”)) may have a negative impact on the ability to drive vehicles or operate machinery. Patients experiencing these adverse reactions are advised to refrain from driving vehicles or operating other machinery until these symptoms disappear completely.
Maltofer® Fol
Suction
Iron from iron(III) hydroxide polymaltose is absorbed according to a controlled mechanism. The increase in serum iron after drug administration does not correlate with total iron absorption measured as incorporation into hemoglobin (Hb). Studies with radiolabeled iron(III) hydroxide polymaltose have shown a strong correlation between iron incorporation into red blood cells and iron content throughout the body. The maximum activity of absorption of iron from iron (III) hydroxide polymaltose is observed in the duodenum and small intestine. As with other oral iron supplements, the relative absorption of iron from iron(III) polymaltose hydroxide, measured as incorporation into hemoglobin, decreases with increasing iron doses. In addition, a correlation was observed between the severity of iron deficiency (specifically, serum ferritin concentration) and the relative amount of iron absorbed (i.e., the more severe the iron deficiency, the better the relative absorption). In anemic patients, iron absorption from iron(III) polymaltose hydroxide, in contrast to iron salts, was increased in the presence of food.
Folic acid is absorbed primarily in the gastrointestinal tract, in particular in the duodenum and small intestine. When taking folic acid at a dose of 0.35 mg, absorption is about 80%.
Distribution
The distribution of iron from iron(III) hydroxide polymaltose after absorption was studied in a study using the dual isotope technique (55Fe and 59Fe). The maximum concentration of folic acid in the blood is achieved after 30-60 minutes. In a single-dose study in 12 healthy women, folic acid from Maltofer® Fol chewable tablets (100 mg iron, 0.35 mg folic acid) was shown to be rapidly absorbed, with a peak plasma folate concentration of 11 ng/ml , is achieved 0.75 hours after taking the drug. Folic acid intensively binds to blood plasma proteins, penetrates the blood-brain barrier (BBB), the placenta and into breast milk.
Biotransformation
Absorbed iron binds to transferrin and is used to synthesize hemoglobin in the bone marrow or is stored, mainly in the liver, where it binds to ferritin.
Folic acid is metabolized in the cells of the small intestine and liver, as well as in other organs. After this, folates bound to transport proteins are distributed to all organs.
Removal
Unabsorbed iron is excreted in the intestines (with feces).
Excretion of folic acid occurs mainly by the kidneys, as well as through the digestive tract.
Folic acid is removed by hemodialysis.