Modern life is characterized by high rhythm, as well as increased mental and physical stress. Lack of sleep, constant stress and other negative factors impair memory and reduce concentration. This leads to a deterioration in the quality of life in general. With age, the risks of developing negative deviations increase. The nootropic drug Semax will help stop age-related changes, increase performance and improve memory.
Nosological classification (ICD-10)
- F07.2 Post-concussion syndrome
- F43.9 Reaction to severe stress, unspecified
- F81 Specific developmental disorders of learning skills
- G46 Vascular cerebrovascular syndromes in cerebrovascular diseases
- H47.2 Optic atrophy
- I69 Consequences of cerebrovascular diseases
- R41.8.0* Intellectual-mnestic disorders
- R45.7 State of emotional shock and stress, unspecified
- R53 Malaise and fatigue
- T66 Unspecified radiation effects
- T90.5 Consequences of intracranial injury
- Z73.0 Overfatigue
- Z73.2 Lack of rest and relaxation
Dosage
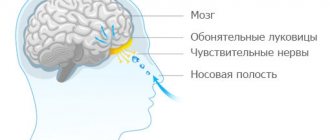
Semax is used intranasally, using a bottle sealed with a plastic screw cap or a dropper cap.
The volume of 1 drop is 0.05 ml (50 µl). 1 drop of standard solution contains 500 mcg of active substance.
For
moderate
stroke , 2-3 drops are administered into each nostril at one time, which is 2000 (4 drops or 0.2 ml)-3000 mcg (6 drops or 0.3 ml). Instillation is carried out 3-4 times a day with an interval between instillations of 3-4 hours. The daily dose is 6000 (12 drops or 0.6 ml) - 12,000 mcg (24 drops or 1.2 ml).
For severe stroke
At one time, 3-4 drops are administered into each nostril, which is 3000 (6 drops or 0.3 m)-4000 mcg (8 drops or 0.4 ml). Instillation is carried out 4-5 times a day with an interval between instillations of 2.5-3 hours. The daily dose is 12,000 (24 drops or 1.2 ml) - 20,000 mcg (40 drops or 2.0 ml).
The drug is prescribed daily for 10 days.
Mode of application
If the bottle is sealed with a plastic screw cap:
—
For initial use, you must remove the plastic screw cap and replace it with the supplied pipette with cap;
- draw the drug into a pipette;
— slightly tilting your head to the side, squeeze out the required number of drops of the drug onto the mucous membrane of the nasal passage.
If the bottle is sealed with a dropper cap:
—
it is necessary to carefully cut off the tip of the pipette and tightly close the pipette with the cap;
— before use, you should turn the bottle over so that the liquid fills the entire space of the pipette;
- remove the cap and squeeze out the required number of drops of the drug onto the mucous membrane of the nasal passage.
The drug should be kept tightly closed with a capped pipette or plastic screw cap or dropper cap.
Indications for Semax solution 0.1%
Intellectual-mnestic disorders in vascular lesions of the brain, conditions after traumatic brain injury and neurosurgical operations; prevention and treatment of post-anesthesia and asthenoneurotic disorders of various origins, incl. after exposure to ionizing radiation; to increase adaptation in extreme situations; prevention of mental fatigue during monotonous operator activity during stressful periods of work and in emergency situations.
Ophthalmology: optic nerve atrophy, neuritis of inflammatory, toxic-allergic and vascular etiology.
In pediatrics: as a nootropic agent in children aged 5 to 14 years in the treatment of minimal brain dysfunction (including ADHD - attention deficit hyperactivity disorder).
Drug interactions
Pharmaceutical
Based on the chemical structure of the drug, the presence of chemically incompatible combinations is not expected: the drug is quickly destroyed and does not enter the gastrointestinal tract.
Pharmacokinetic
Considering the chemical structure of the drug (heptapeptide is a synthetic analogue of ACTH, completely devoid of hormonal activity), the rate of absorption and rate of entry into the blood, as well as the intranasal route of administration, the influence of other drugs on the pharmacokinetic parameters of the drug Semax nasal drops 1% is not expected.
Considering the method of administration of Semax nasal drops 1%, it is undesirable to administer agents that have a local vasoconstrictor effect when administered intranasally.
Directions for use and doses
Intranasally. One drop contains 50 mcg of heptapeptide. 2-3 drops (no more) in each nasal passage. If it is necessary to increase the dose, drops are instilled in several doses at intervals of 10–15 minutes. Single dose - 200-2000 mcg (3-30 mcg/kg), daily dose - 500-5000 mcg (7-70 mcg/kg).
The drug is prescribed daily for 3–5 days; if necessary, the course of treatment is extended to 14 days.
For diseases of the optic nerve - 2-3 drops in each nasal passage 2-3 times a day. The daily dose is 600–900 mcg/day. The course of treatment is 7–10 days. In addition, it is possible to administer by endonasal electrophoresis from the anode: current strength is 1 mA, duration of exposure is 8–12–15 minutes. The daily dose is 400–600 mcg/day. The course of treatment is 7–10 days.
In pediatrics: for minimal brain dysfunction, the drug is instilled 1-2 drops into each nasal passage 2 times a day (morning and afternoon). The daily dose is 200–400 mcg/day. The course of treatment is 30 days.
pharmachologic effect
Semax nasal drops 1% is an original synthetic peptide drug, which is an analogue of the ACTH4-10 fragment (methionyl-glutamyl-histidyl-phenylalanyl-prolyl-glycyl-proline), completely devoid of hormonal activity.
All amino acids are L-form. The drug has an original mechanism of neurospecific action on the central nervous system. It is a synthetic analogue of corticotropin, which has nootropic properties and is completely devoid of hormonal activity. The drug improves cognitive processes of the brain, such as memory, learning, attention; increases mental performance. Improves the adaptation of brain cells to hypoxia, anesthesia and other damaging influences.
The drug is practically non-toxic with single and long-term administration. Does not exhibit allergic, embryotoxic, teratogenic and mutagenic properties. Does not have a local irritant effect.
Impact
Semax drops, the instructions confirm this, have a neurometabolic effect on the central nervous system when used in small doses. When used, it is possible to improve attentiveness and stimulate memory development. This allows you to quickly assimilate new information, which means increasing learning rates. In addition, it reduces the body’s negative reactions during oxygen starvation or cerebral ischemia.
The neuroprotective effect of the drug allows you to stop and limit areas of brain damage. As a result, it is possible to increase the effectiveness of restorative therapy for various pathological processes.
With increasing doses of Semax nasal drops, they additionally have antioxidant, angioprotective and antihypotoxic effects. This minimizes the development of problems due to oxygen starvation caused by decreased blood flow to the brain.
The active substance is absorbed from the nasal mucosa at rates of 60-70%. It spreads quickly throughout the body. Therefore, a positive result is obtained even after a single dose in the shortest possible time.
Due to the simple chemical structure of the drug, incompatible combinations with other drugs are excluded. The active substance does not enter the bloodstream through the gastrointestinal tract.
results
The influence of Semax use and the time of initiation of rehabilitation on the level of BDNF
At study entry, plasma BDNF levels were low and did not differ between the early and late rehabilitation groups (see Tables 1 and 3).
Table 3. Dynamics of BDNF levels in the examined patients (M±m) Note. * - differences are significant compared to the initial indicators at t≥2.0 and p≤0.05; ** - tendency towards significant differences compared to the initial indicators at t=2.03; and p=0.052; *** — differences are significant between subgroups 1a and 1b at t=2.62 and p=0.011; # - differences are significant between subgroups 1a and 1b at t=3.44 and p=0.001; ## — differences are significant between subgroups 2a and 2b at t=4.86 and p=0.000; ### — differences are significant between subgroups 2a and 2b at t=4.12 and p=0.000; † — differences are significant between subgroups 1b and 2b at t=2.40 and p=0.021. 1 month after the start of rehabilitation, the level of BDNF in all subgroups, with the exception of subgroup 2b, significantly increased compared to the initial values and reached an average level (Table 3). After 5 months, the level of BDNF in subgroups 1a, 1b and 2a decreased compared to the end of the 1st month, but remained significantly higher than the initial values. In subgroup 2b, at the end of the 1st month, there was a tendency towards a significant increase in the level of BDNF (see Table 3), which disappeared by the end of the observation, and the level of BDNF in this group remained low throughout the study.
Treatment with Semax influenced the level and dynamics of BDNF; the differences between groups 1 and 2 were significant throughout the study. In addition, the administration of Semax eliminated the effect of the time of initiation of rehabilitation on the level and dynamics of BDNF. Thus, in subgroups 1a and 2a, the dynamics and magnitude of the increase (Δ) of BDNF did not differ from each other, but were significantly different from subgroups 1b and 2b. After 1 month from the start of observation in subgroups 1a and 2a, the increase in BDNF reached 191.3 and 187.7% relative to the initial level, while in subgroups 1b and 2b it was 112.8 and 56.9%, respectively, which caused significant differences between subgroups. After 5 months, the differences remained: with early rehabilitation in subgroup 1a, the increase in BDNF was 150.1% relative to the initial level, in subgroup 1b - 45.3%, and with late rehabilitation - 154.3 and 35.4%, respectively ( see table 3). In addition, in subgroup 1a, its level from the end of the 1st month to the end of the study decreased by 20.8% (3.1 ± 1.7 pg/ml), while in subgroup 1b the decrease was 57.7% ( 5.6±1.3 pg/ml; t
=2.92;
p
=0.011).
In subgroup 2a, the level of BDNF from the 1st to the 5th month decreased by 22.4% (2.8±2.5 pg/ml), in subgroup 2b it decreased by 50.0% (1.4±1.5 pg/ml; t
=2.25;
p
=0.029).
In patients who did not receive Semax, the magnitude of the increase in BDNF levels depended on the time of initiation of rehabilitation. Thus, in subgroup 1b after 1 month there was a significantly greater absolute and relative increase in BDNF than in subgroup 2b - 112.8 and 56.9%, respectively. By the end of the observation period, the differences between these subgroups remained: 41.9 and 28.7% (a tendency towards significant differences at t
=1.81;
p
=0.087) (see Table 3).
Thus, the use of Semax neutralized the effect of the start time of rehabilitation on BDNF indicators. The drug contributed to a more rapid increase in BDNF levels in the 1st month of rehabilitation and maintained a high and uniform concentration throughout the entire observation period. In subgroups 1b and 2b, the dynamics and increase in BDNF depended on the time of initiation of rehabilitation, and early rehabilitation led to a larger and more rapid increase in BDNF levels.
The influence of the use of Semax and the timing of the start of rehabilitation on the severity of motor disorders
When included in the study, 43 (71.7%) patients of the 1st group and 37 (74.0%) patients of the 2nd group had motor disorders in the form of hemiparesis, while their initial severity did not differ between groups (see. Table 1). When analyzing the influence of the start time of rehabilitation on the dynamics of motor disorders, it was found that in subgroups 1a and 1b, after 1 month there was a statistically significant increase in strength in the lower and upper extremities (Table 4),
Table 4. Dynamics of muscle strength recovery in the examined patients (M±m) Note.
* - differences are significant compared to the initial value at t≥2.01 and p≤0.05; ** — differences are significant between subgroups 1a and 2a at t=2.51 and p=0.015; *** — differences are significant between subgroups 1b and 2b at t=2.41 and p=0.02; # - tendency towards significant differences between subgroups 1a and 2a at t=1.75 and p=0.081; ## — differences are significant between subgroups 1a and 2a and 1b and 2b at t≥2.99 and p≤0.005. which persisted throughout the study. In subgroups 2a and 2b, a significant increase in strength in the limbs was observed only at the end of the observation. In subgroups 1a and 1b, the increase in hand strength by the end of the 1st month was significantly ahead of similar indicators in subgroups 2a and 2b. Thus, 1 month after the start of rehabilitation in subgroups 1a and 1b, muscle strength in the upper limb was restored by 89.4 and 97.8%, respectively, in relation to the final assessment, while in subgroups 2a and 2b the recovery was at a similar time point in relation to the final assessment was only 54.2 and 47.3%. Final recovery in the arm was also better with early rehabilitation (tendency toward significant differences at t
< 1.92 and
p
< 0.072).
In the lower limb, early rehabilitation did not affect the dynamics of strength gain in the 1st month, but led to better final recovery (see
Table 4).
A comparison of the intensity of recovery of movements in the arm and leg showed that in the 1st month, regardless of the time of the start of rehabilitation, movements in the leg were restored significantly slower compared to the arm ( p
= 0.027). When analyzing the effect of Semax on the degree and dynamics of reduction in the severity of paresis, no significant differences were noted between groups 1 and 2 throughout the study.
The results of studying the effect of the use of Semax and the level of BDNF on the dynamics of paresis, taking into account the time of the start of rehabilitation, turned out to be interesting. The assessment was carried out in two stages. At the first stage, the results of observation of subgroups 1a (early rehabilitation, taking Semax) and 2a (late rehabilitation, taking Semax) were analyzed. This choice was due to the fact that the initial and subsequent levels and dynamics of BDNF throughout the study between these subgroups were completely comparable, in contrast to the levels and dynamics of BDNF between subgroups 1b and 2b (see Table 3). Despite the same level of BDNF and the administration of Semax in both subgroups, in subgroup 1a (early rehabilitation) faster rates and better final recovery were noted (see Table 4). At the second stage, a comparison was made of subgroup 1b with subgroup 2a. At baseline, BDNF levels did not differ between these subgroups (see Tables 1 and 3). By the end of the 1st month and by the end of the study, the level of BDNF and its increase were significantly greater in subgroup 2a ( t
>2.43;
p
<0.018).
At the same time, the rate of decrease in paresis in the arm by the end of the 1st month was significantly higher in subgroup 1b ( t
= 2.67;
p
= 0.01). The data obtained can be interpreted as evidence of a more pronounced effect of even lower concentrations of BDNF on recovery, in particular on the reduction of paresis in the early period after I.I. This feature is most likely associated with the dynamics of plasticity processes after focal brain damage and with the presence of an optimal time period in the early period for the action of trophic factors, including BDNF [3, 11].
Thus, the early start of rehabilitation contributed to a faster and more complete reduction of paresis in the arm and leg, which is most likely due to the optimal state of plasticity processes in the brain during this period of time for the restoration of muscle strength and simple movements.
The influence of Semax use and the time of initiation of rehabilitation on the ability to self-care
At baseline, the Barthel score did not differ between groups (see Table 1). 1 month after the start of rehabilitation, the increase in the sum of points in subgroups 1a, 1b and 2a was statistically significant (Table 5).
Table 5. Dynamics of the sum of points on the Barthel scale in the examined patients Note. * - differences are significant in groups between indicators at inclusion, after 1 and 5 months at t>2.61; p<0.026; ** — differences are significant between subgroups 1a and 1b at t=2.84 and p=0.006; *** — differences are significant between subgroups 2a and 2b at t=4.48 and p=0.000; # - differences are significant between subgroups 2a and 2b at t=2.07 and p=0.044; ## — differences are significant between subgroups 1a and 2b at t=2.08 and p=0.042; † — differences are significant between subgroups 1b and 2b at t=2.36 and p=0.022; †† — differences are significant between subgroups 1b and 2b at t=2.39 and p=0.021. By the end of the 5th month, a further increase in the sum of points was observed and the increase became statistically significant in all subgroups.
The administration of Semax influenced the increase in values on the Barthel scale, significantly accelerating it in the 1st month of rehabilitation. Thus, in subgroups 1a and 2a by the end of the 1st month of rehabilitation, the increase in the sum of points was 78.5 and 62.4% of the total increase, while in subgroups 1b and 2b the increase was only 43.8 and 21.9% . In addition, the administration of Semax increased the total score on the Barthel scale during late rehabilitation (see Table 5).
When analyzing the effect of Semax on the dynamics of individual sections of the Barthel scale, it was noted that in subgroup 1a, statistically significant dynamics by the end of the 1st month was due to an increase in scores on the movement criteria (the number of points on the “climbing stairs” test increased by 46%, “transplanting " - by 40%, "walking" - by 80%) and self-care ("eating" + 54%, "bathing" + 60%, "using the toilet" + 46%). In subgroup 2a, similar dynamics were observed: the maximum increase in indicators was recorded in the following sections: “climbing stairs” (+32%), “transplanting” (+40%), “walking” (+77%), “eating” (+49 %) and “using the toilet” (+38%).
Another important factor that, along with the use of Semax, influenced the dynamics of indicators on the Barthel scale was the time of the start of rehabilitation measures. Early rehabilitation (subgroups 1a and 1b) was accompanied by a faster increase in the sum of points by the end of the 1st month compared to subgroups 2a and 2b, as well as a larger final increase in subgroup 1b compared to subgroup 2b (see Table 5).
The total Barthel score correlated with BDNF levels at the end of the 1st month and at the end of the study ( r
=0.31 and
r
=0.24, respectively;
p
<0.017).
This correlation was more pronounced with early rehabilitation compared to late ( r
=0.37 and
r
=0.21, respectively;
p
<0.011), as well as at the end of the 1st month compared to the end of the study (
r
=0.36 and
r =
0.19, respectively;
p
< 0.047).
Thus, the administration of Semax both during early and late rehabilitation significantly accelerated the increase in Barthel scale scores by the end of the 1st month, and also contributed to the achievement of better final recovery during late rehabilitation. There was a correlation between BDNF levels and Barthel recovery, more significant with early rehabilitation, indicating that there is an optimal time window for BDNF to act.
Contraindications and negative reactions of the body
The main contraindications for Semax drops are hypersensitivity to the active component. The medicine is not prescribed to children under 7 years of age. Its use is contraindicated when carrying a child and during breastfeeding. This is due to the fact that studies have not been conducted to prove safety for the fetus and newborn child.
It is necessary to stop using the drug if you confirm:
- Acute psychotic disorders, which are characterized by difficulties assessing reality.
- Conditions of internal anxiety that cause dizziness, panic, muscle tension, shortness of breath.
- History of seizures, characterized by involuntary contraction of muscle groups.
There were no side effects when taking the medication. Sometimes minor irritation of the mucous membrane may occur. Signs of overdose were not affected even with repeated dosage increases.
You can find both positive and negative reviews about the drug. Many patients emphasize that after the course of treatment, memory and concentration have significantly improved. Many also note a positive effect associated with slowing the progression of dementia.
All negative reviews, as a rule, contain information that there is no positive result. Negative side reactions of the body are also often observed, due to which it was necessary to abandon the medicine.