Structural features
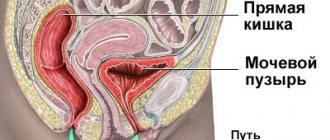
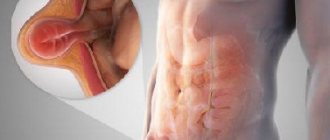
The structure of the urethra, which is called the urethra, differs according to a particular gender:
- In women, the urethra is short - 3-4 cm and wide - 1-1.5 cm.
- In men, the urethra is long – up to 30 cm and narrow – up to 8 mm. In addition, it has curves.
The anatomical features of the structure of the organ are the reason why women often develop cystitis. This is explained by the fact that the infection quickly penetrates the bladder through the short urethra and causes inflammation. In men, the urethra is connected to the vas deferens, so the secretion of the sex glands is also excreted through the urethra.
The inner surface of the urethra is covered with mucous membrane. When it becomes inflamed, which can be caused by various reasons, urethritis develops. In women, this pathology does not lead to disruption of the outflow of urine, which eliminates the occurrence of severe discomfort and speeds up treatment of the disease. Due to the anatomical features, the infection in the male urethra lingers for a long time, causing severe swelling of the mucous membrane. This leads to disruption of the outflow of urine, and in severe cases to painful retention of urination.
How is urethral stricture treated?
Treatment of urethral stricture in men and women can be different:
- Radical. These are urethroplasty operations that are considered the most effective.
- Non-radical. Such operations most often provide a temporary effect and are accompanied by frequent relapses.
Radical methods for removing urethral stricture include:
- Anastomotic urethroplasty – excision of the area of stenosis and subsequent connection of urethral fragments.
- Replacement urethroplasty is the replacement of part of the lumen with the patient’s own mucous membrane.
- Perineal urethroplasty – removal of the urethral opening into the perineum.
Non-radical methods of treating urethral stricture:
- bougienage (temporary restoration of the lumen of the urethra)
- urethrotomy (dissection of urethral stricture);
- stenting (placement of a stent in the lumen of the urethra).
At the State Urology Center you can get qualified help from a urologist and get rid of such a defect and its unpleasant symptoms. Here you will undergo comprehensive diagnostics and treatment, and also receive clinical recommendations for combating urethral stricture in order to avoid its complications. To receive medical services, you only need to make an appointment with a urologist at a time convenient for you.
On weekdays you can get an appointment with a urologist on the day of your visit
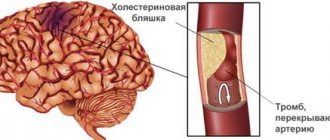
Causes of inflammation
Understanding what the urethra is in women and men, one can understand how inflammatory processes occur in the organ. It can be caused by infectious and non-infectious factors.
Non-infectious urethritis often occurs with the development of urolithiasis. In this case, small stones move with the urine current, which can damage the mucous membrane of the urethra, which causes inflammation. Also the cause of the development of non-infectious urethritis are:
- Injuries. They most often occur during cystoscopy and catheterization.
- Tumors. Malignant neoplasms always cause inflammation of the mucous membrane.
- Allergy. Negative body reactions can be caused by negative reactions of the body to certain foods or substances.
Inflammatory processes in men can occur when the urethra is narrowed due to prostatitis or benign prostatic hyperplasia. In such cases, urinary stagnation occurs.
Non-infectious urethritis must be treated in the early stages of development. To do this, on the recommendation of a doctor, you should buy the necessary medications at the pharmacy to relieve inflammation. If therapy is not carried out quickly, infection may occur and complications may arise.
Infectious urethritis can be caused by various pathogens. Before treating the disease, it is necessary to conduct research aimed at determining the type of pathogen. Nowadays you can buy various medications in pharmacies that will quickly overcome the most severe infection. But at the same time, it is necessary to urgently take the required measures. The infection that causes the development of urethritis can enter the body sexually or hematogenously.
Female urethral surgery
A.V. Kurenkov Candidate of Medical Sciences, Associate Professor of the Department of Urology and Andrology of St. Petersburg MAPO
A detailed report on surgical methods for treating urethral diseases was made by an employee of the Department of Urology of Northwestern State Medical University named after. I.I. Mechnikova Doctor of Medical Sciences Alexander Viktorovich Kurenkov.
Relevance of the problem
According to the speaker, when it comes to urethral surgery in men, some aspects are rethought every year and innovations are introduced, but for women the topic is not covered enough. At the same time, there are a number of diseases that require urethral surgery in women: urethral diverticulum, paraurethral leiomyoma, urethral dilatation in women, strictures, stress urinary incontinence and, finally, urethral cancer. In addition, surgical intervention may be required in the presence of urethro-vaginal fistulas.
Urethral diverticula in women: statistics, causes, symptoms
One of the diseases in this series is urethral diverticulum. It was first described by William Hay in 1805. Diverticula originate from the urethral wall and contain fibrous tissue with urethral epithelium. Chronic inflammation leads to loss of the epithelial layer and fixation of the diverticulum wall to the underlying periurethral fascia and the anterior vaginal wall.
Urethral diverticulum in women is a fairly rare disease: its prevalence varies from 0.6% to 4.7%. However, the etiology of the disease has been discussed for more than 200 years and continues to remain a controversial issue. Today, two theories of the origin of urethral diverticula in women prevail: one suggesting a congenital nature of the disease (not confirmed by extremely rare observations of the disease in pediatric practice) and an infectious nature (seems more logical).
With a diverticulum, obstruction of the excretory ducts of the paraurethral glands leads to their inflammation and abscess formation. The resulting cavity can break into the lumen of the urethra as a result of trauma (coitus, vaginal birth) or tissue necrosis and become a urethral diverticulum or remain without connection with the urethra, forming a paraurethral cyst when the acute phase of inflammation is over. A diverticulum with urine stasis inside the cavity creates a favorable environment for the development of the inflammatory process, which explains the possibility of re-infection of the diverticulum.
Among malignant neoplasms of urethral diverticula, adenocarcinoma prevails, which also confirms the hypothesis of the origin of diverticula from “occluded” paraurethral glands. Complications of urethral diverticula include infections (acute or chronic urethritis), the formation of stones in the diverticulum cavity, and malignancy. It is extremely rare and does not exceed 5% of all urethral tumors. The histological structure of neoplasms of urethral diverticula in women is dominated by adenocarcinoma (61%), transitional cell carcinoma (27%) and squamous cell carcinoma (12%). Among the clinical manifestations of the disease, the classic “three D” complex stands out: dysuria, dyspareunia and dribbling (urine leakage after urination). However, this combination is not quite common: symptoms such as urgency, pollakiuria, urinary incontinence and pain predominate. The results of published studies made it possible to estimate the percentage of symptoms occurring with urethral diverticulum in 627 women. In first place in terms of frequency of occurrence is pollakiuria (56%), dysuria is almost as pronounced (55%), followed by recurrent infections (40%) and palpable painful tumors in the urethral area (35%), followed by stress incontinence (32 %) and urinary leakage (27%), urgency (25%), hematuria (17%), dyspareunia (16%), purulent urethral discharge (12%) and urinary retention (4%). The disease is asymptomatic only in 6% of cases.
Diagnosis of urethral diverticula
The physical examination of patients with diverticulum involves inspection and palpation of the anterior vaginal wall, during which a painful or painless mass may be detected with the discharge of pus, urine or blood from the external urethral meatus after pressing on the area of the diverticulum. It is worth considering that the size of diverticula does not correlate with the presence of symptoms, however, with large (more than 1 cm) diverticula, a painful formation is almost always detected during vaginal examination. Consolidation in the area of the diverticulum is suspicious in terms of malignancy or the presence of a stone in the diverticulum. It is also necessary to carry out differential diagnosis: in addition to urethral diverticulum, the causes of palpable formation in the periurethral area can be diseases that are divided into two categories: embryonic (Skeene's gland abscess, Gartner's duct cyst, ectopic ureterocele, as well as vaginal wall cyst) and neoplastic (paraurethral myoma and leiomyoma, urethral carcinoma, hemangioma, urethral varicocele, endometriosis and metastases to the vaginal wall).
In some cases, it is useful to perform urethroscopy during diagnosis. This requires a so-called “female urethroscope” with a circular cuff that prevents leakage of fluid. Against the background of the inserted urethroscope, palpation is performed to determine the location, size and consistency of the diverticulum. The orifice of the diverticulum can be visualized by applying pressure to the area of the diverticulum on the anterior vaginal wall. In this case, the contents of the diverticulum (pus, blood, cloudy urine) are released into the lumen of the urethra from the anastomosis. If a urethral diverticulum is suspected, excretory urography is indicated, which will exclude ectopic ureteral orifice.
A method such as voiding cystography is now a thing of the past. Before the introduction of magnetic resonance imaging (MRI), it was considered by many as the best method capable of confirming the presence, size and shape of a urethral diverticulum. Currently, voiding cystography is no longer considered the method of choice for diagnosing urethral diverticula because it does not have the same sensitivity as MRI. Frequent false-negative results when using this method are due to incomplete filling of the diverticulum due to low urine flow rate, localization (multi-chamber) of the diverticulum and a narrow entrance to the diverticulum.
The diagnosis also uses retrograde positive pressure urography, which uses double-balloon Davis or Trattner catheters: they are installed in the urethra so that the distal balloon is located in the area of the bladder neck. The distal and then the proximal balloon are inflated by injecting 20–30 ml of water, saline or contrast agent into them. After the balloons block both ends of the urethra, 4–7 ml of a 30% contrast agent solution is administered through the third channel. It enters the urethra through an opening located in the area between the balloons. The contrast agent injected under pressure flows around the catheter and fills the diverticulum cavity. Retrograde positive pressure urethrography can also be performed using a modified Foley catheter (due to the low availability of Davis and Trattner catheters in Russia).
Ultrasound examination can detect both cysts and urethral diverticula, but differential diagnosis of these diseases without visualization of the diverticulum opening is impossible. Thus, if a fluid formation is detected posterior to or inferior to the bladder, the possibility of urethral diverticulum should be considered.
Today, MRI is considered the best method for identifying and imaging urethral diverticula in women. It allows, better than any other method, to obtain an image of soft tissue structures and details of the anatomical structure of the area under study, even if the diverticulum has a multi-chamber structure and is not connected to the lumen of the urethra by a clearly defined anastomosis. In addition, MRI is a non-invasive method because it does not require urethral catheterization. Compared with the surrounding tissue, urethral diverticula appear as a high-intensity magnetic resonance signal on T2-weighted images and a low-intensity magnetic resonance signal on T1-weighted images.
Classification of urethral diverticula
In clinical practice, the classification of urethral diverticula using the L/N/S/C3 system is used, which allows the clinician to evaluate all factors related to the presence of a urethral diverticulum before diverticulectomy. In this system, L is localization (distal, middle, proximal third of the urethra with or without extension towards the bladder); N – number (single diverticulum or multiple); S – size (in centimeters in two dimensions); C1 – configuration (simple, multi-chamber, “folded”); C2 – communication with the urethra (proximal, middle, distal third); C3 – urinary retention.
Treatment of urethral diverticula
Surgical treatment – diverticulectomy – is recommended for patients with symptomatic diverticulum and/or large diverticulum. Correction of stress urinary incontinence at the same time as diverticulectomy is not recommended due to the high risk of recurrent incontinence, obstruction, or urethral erosion. The likelihood of developing stress urinary incontinence is assessed using urodynamic studies and localization of the diverticulum. The treatment for stress urinary incontinence after diverticulectomy is a transvaginal sling. This operation is performed no earlier than 3 months after excision of the diverticulum.
In addition, there are alternative treatments for diverticulum. In 1979, a method was described (Lapides J.), which consists of “opening” the urethral diverticulum into its lumen using a knife-electrode. The modified Lapides technique uses a 10 Charrière pediatric resectoscope with a Collins knife. Endoscopic diverticulectomy is indicated for patients with primary or recurrent diverticulum located in the distal urethra. The purpose of the operation is to create a wide diverticulum anastomosis with the lumen of the urethra for free drainage of the diverticulum cavity. However, endoscopic treatment of urethral diverticula located in the middle and proximal portions of the urethra is not recommended due to the high risk of developing urinary incontinence after surgery.
In addition, in 1970, Spence and Duckett described a method for marsupilization of a diverticulum through transvaginal dissection of all underlying tissues in the projection of the mouth of the diverticulum. The vaginal and urethral mucosa are approximated with a continuous absorbable suture, leaving the Foley catheter in the bladder for 2–3 days. This method of treatment is permissible only when diverticula are localized in the distal third of the urethra, since marsupilization of diverticula located in the middle and proximal part of the urethra also ends in the development of urinary incontinence in almost 100% of cases. Other complications of marsupilization of diverticula include recurrence, “vaginal” urination, and splashing of the urine stream when urinating.
It is also worth mentioning rare methods of treating diverticula, including incision and filling the diverticulum cavity with cellulose (Oxygel, Gelfoam), partial ablation of the diverticulum and transvaginal suturing of the diverticulum mouth.
During a diverticulectomy, a U-shaped incision is made and the anterior vaginal wall is dissected. After transverse incision of the periurethral fascia, the anterior and posterior layers are mobilized, exposing the underlying diverticulum. In this case, you need to reach the mouth of the diverticulum and open the urethra: then the chances of avoiding a relapse increase. To prevent the formation of urethrovaginal fistulas, the excised tissue is sutured in various directions.
Complications of urethral diverticula
It must be remembered that if its wall or anastomosis area is incompletely excised, the urethral diverticulum may recur. However, recurrence can be avoided through detailed preoperative assessment and a clear understanding of the structure of the diverticulum (L/N/S/C3 system). Also, to prevent relapse, it is necessary to suture the wound without the formation of “dead” spaces and cavities.
The presence of a relapse is determined by complaints, palpable formation under the urethra and recurrent urinary tract infection. The Martius flap is used to treat recurrent diverticula. Distal recurrent diverticula of small sizes can be successfully eliminated using the transurethral method or the Spence operation (marsupilization).
Urethrovaginal fistulas are a well-known complication of surgical treatment of urethral diverticula. The reason for the formation of fistulas is a violation of the surgical technique: making vertical incisions in the mucous membrane of the vagina and urethra and suturing the wound of the urethra and vagina in one direction. Suturing of the urethrovaginal fistula is recommended no earlier than after 4 months using a Martius flap.
As an example, the speaker shared his own observations: from 1991 to 2015, 31 patients were observed with diverticula at an average age of 37.8 years (19–59 years). 23 women had multilocular diverticulum. Localization – in 18 patients in the proximal urethra, in 10 – in the middle and in 3 – in the distal. Only one of the patients had a stone in the diverticulum. Symptoms were dominated by dysuria (72.2%), dyspareunia (50%), perineal pain (45.5%) and recurrent urinary tract infections (40.9%). The least common occurrence – in 5.2% – was post-micturition leakage. 28 patients (90.3%) underwent diverticulectomy, and 3 (9.7%) underwent marsupilization. Diverticulum recurrence was observed in 3 patients (9.7%), urinary incontinence in 4 (12.9%), two of them underwent midurethral sling.
Urethral tumors
Another disease requiring surgical treatment is paraurethral leiomyoma. This is a rare benign mesenchymal tumor consisting of smooth muscle fibers of the paraurethral region. It was first described by Büttner in 1894. It is extremely important to understand that this neoplasm does not become malignant. As a rule, the site of localization is the proximal urethra. Clinical manifestations of the disease are recurrent UTI, difficulty urinating, dysuria and a palpable mass on vaginal examination. The diagnostic complex includes uroflowmetry with determination of residual urine volume, cystourethroscopy, ultrasound + transvaginal needle biopsy, as well as MRI (isohypointense image on T1-weighted images and isohyperintensity on T2-weighted images.
Due to the rarity of the disease, there is no clear treatment protocol, but in 2004 it was suggested (Kobayashi T.) that leiomyoma is an estrogen-dependent tumor. Typically, urethral leiomyomas increase during pregnancy, when estrogen levels are elevated, and regress in the postpartum period (Bhandarkar DS et al., 1992). Due to the possibility of hormonal influence, conservative treatment with gonadotropin-releasing hormone (GnRH) analogues has been described (Tantbirojn P. et al., 2006).
Strictures and dilatation of the urethra in women
Next, the speaker touched upon the topic of urethral strictures in women and urethral dilatation, one of the most controversial procedures in urogynecology. In 1999, in a survey of urologists in the USA (Texas), 21% of specialists with more than 10 years of experience considered urethral dilatation in women to be a very successful method of treating urethral syndrome, but at the same time, none of the 42 urologists with less experience considered this method useful (Lemack GF et al.). The seventh edition of Campbell's Urology (Walsh PC et al., 1998) states that "there is no objective evidence to support the benefit of this form of treatment." In subsequent editions of the source, these provisions and wording have not changed. However, when nationally representative data on urethral dilatation in women were assessed in the United States (1992–2000), it was found that this procedure was performed as frequently as bougienage in men with urethral strictures (Santucci RA et al., 2008).
The most popular method of urethral dilatation in women is dilatation of the urethra with Hegar metal bougies (also used for cervical dilatation) and Van Buren bougies. In rare cases, the Otis knifeless urethrotome is used. Less traumatic, especially for the mucous membrane, is the method of balloon dilatation, in which a slow and gradual expansion of the urethra occurs. This is confirmed by the observation of 4 women who performed urethral dilatation with a balloon catheter independently every day for 3 months while maintaining urethral patency (Zimmern PE, 1997).
The size of the external opening of the female urethra: what is considered normal?
The question of to what extent to dilate the urethra is extremely important. As early as 1936, it was noted that if the “caliber” of the external opening of the urethra is less than 30 F, relapses of lower urinary tract infections are inevitable (Stevens WE, 1936). Later it was indicated that the “normal female urethra” should have a lumen >24 F, then such patients would not have signs of recurrent UTI (McCannell DA, Halie W., 1982). Some authors recommend urethral hyperdistension to 45 F, which they claim will give better results compared to standard dilatation (Essenhigh DM et al., 1968, Park JM et al., 2001). A 2012 publication indicates that dilatation to 41 F, accompanied by meatus tears, is a necessary measure for the treatment of distal urethral constrictions (Romman AN et al., 2012).
Today, there are several publications that attempt to establish normal values for the lumen of the female urethra. Thus, it was noted that in girls under 14 years of age, the lumen of the external urethral opening averages 14 F (Graham JB et al., 1967). Further, before the onset of puberty, there is a gradual and age-proportional increase in the lumen of the urethra from 14 F to 22 F (Immergut MA, Wahman GE, 1968). The average normal caliber in 250 women aged 20–90 years was 22 F in all age groups (Uehling D, 1978)
The technique for determining the “caliber” of the urethra consists of sequentially introducing bougies of progressive size until resistance is detected when removing the instrument. This maneuver reflects the physician's subjective tactile impression regarding the safe limits of extensibility (reverse stiffness) of the urethra as a static and non-dynamic organ. In an experimental model of a urethra with a length of 5–7 cm and a diameter of ≥10 F, no obstructive flow pattern was observed over the normal range of micturition pressure (Tanagho EA, McCurry E., 1971). In practice in the UK and USA, most urologists dilate the urethra to 32 F, while 86% of pediatric urologists surveyed limit themselves to bougies from 20 to 26 (Masarani M., Wills RG, 2006).
For pain relief during dilatation, the classic recommendation is to use terminal anesthesia with the anesthetic injected into the urethra and exposed for 10 minutes before the procedure (Glenn JF, 1983). In 1980, it was noted (Ostergard DR) that when local anesthesia was used during office urethral dilatation to 38 F, only 10% of patients experienced enough discomfort to require discontinuation of the procedure. It was noted that in the UK up to 90% of urologists used general anesthesia, while the same 90% of their colleagues from Texas (USA) used local anesthesia or did without it at all (Lemack GF et al., 1999).
Causes and clinic of SBU
Stricture urethral disease (SUD) accounts for 4–13% of the etiology of bladder outlet obstruction in women (Nitti VW et al., 1999; Groutz A., Blaivas JG, 2000). As a rule, SBU is the result of urethral surgery in women (diverticulectomy, reconstructive surgery of urethrovaginal fistulas, surgeries for urinary incontinence, TUR of bladder tumors) and very rarely - repeated urethral dilatation procedures (Keegan KA, Nanigian DK, Stone AR, 2008). Also, strictures of the female urethra can be the result of trauma (prolonged labor) or prolonged catheterization of the bladder. Fibrous changes in the mucosa extending to the spongy urethra are a consequence of infectious processes such as gonorrhea and tuberculosis (Stevens WE, 1923), or radiation therapy.
The clinical manifestations of urethral strictures in women do not differ from those of bladder outlet obstruction: a weak urine stream, dribbling, recurrent urinary tract infections and an overactive bladder.
When diagnosing, the presence of a stricture can be confirmed by the inability to pass a Nelaton catheter >12 F (Smith A.L. et al., 2006), and there is also a pathognomonic symptom of the sensation of scar tissue when passing a flexible cystoscope 17 F (Blavias JG et al., 2012). Also, when making a diagnosis, CUDI (pressure/flow study) and voiding cystography are used.
According to an analysis of PubMed and MedLine data, from 1923 to 2000. In the English-language literature, priority was given to urethral dilation if results were satisfactory, and patients were offered prolonged intermittent catheterization with catheters of the appropriate size (Stanton, 1923, Stevens WE, 1923, Takao M. et al., 1992, Smith A.L. et al., 2006). However, since 2000, publications began to appear in which urethral dilatation in women with SBU was seriously criticized. In particular, relapses of the disease are indicated in 16 of 17 patients undergoing urethral dilatation (Blavias JG et al., 2012). However, the authors noted only 2 relapses after urethroplasty.
When talking about urethroplasty, it is necessary to understand that the critical factors for choosing a surgical approach are the location, extent of the stricture, and preservation of urinary continence. Ventral urethroplasty of the labia and vaginal mucosa (distal strictures) and dorsal buccal urethroplasty (extended and proximal strictures) are considered as options.
In general, surgery of the female urethra is an area of urogynecology that is characterized by contradictions and the lack of certain standards in diagnosis and treatment. The main reason for this situation is that surgical diseases of the female urethra are quite rare. Modern publications on the topic either cover small series of patients or are presented by so-called “case reports”: observations of individual cases from practice. Within one speech, the speaker vividly and fully presented to the audience the maximum amount of new and interesting information on the stated topic.
Urology Digest website - Urodigest.ru
Comments
To post comments you must log in or register
Symptoms
At the initial stage of development, urethritis does not manifest itself with severe symptoms. Almost always, men experience slight discomfort in the penile canal, and women experience slight discomfort in the lower abdomen, slightly above the pubis. Such symptoms often go unnoticed.
As a rule, patients consult a doctor when the disease becomes acute. This is manifested by severe pain and burning when urinating. There is also a frequent urge to urinate, but the bladder cannot be emptied completely. In severe cases, the following symptoms are noted:
- Cloudy urine, possible inclusions of blood and mucus;
- Purulent discharge with an unpleasant odor.
In addition, a symptom of inflammation of the urethra is redness:
- In men, the external opening of the urethra.
- In women, the labia majora and minora.
WHAT HORMONES REGULATE THE FUNCTION OF THE MALE REPRODUCTIVE SYSTEM?
The male reproductive system is primarily dependent on three hormones.
Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are produced by the pituitary gland, a gland located at the base of the brain. FSH stimulates sperm formation, LH stimulates testosterone synthesis. Testosterone is necessary for the production of sperm, as well as the occurrence of secondary sexual characteristics: increased muscle mass, male-type body hair growth, sexual attraction to women, deep voice, etc.
Diagnostics
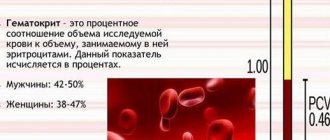
In order to purchase effective medications in order to carry out effective treatment that will quickly restore the condition, you need to conduct a diagnosis. The general analysis of power is indicative. Elevated leukocytes in its results indicate the presence of an inflammatory process. Similar deviations from the norm are also observed during a general blood test.
To confirm the diagnosis, smears from the urethra are examined. Biomaterial is collected from the affected area using a special instrument. For the most effective antibacterial treatment, bacteriological urine culture is performed. With its help, the causative agent of the disease is determined. If a viral infection is suspected, a PCR test is additionally prescribed. Also, on the doctor’s recommendation, various instrumental examinations may be prescribed.
Preparation for the procedure
Before dilating the urethra, a man undergoes a standard set of examinations:
3-5 days before the procedure, you should stop drinking alcoholic beverages and blood-thinning medications (you may need to consult your doctor).
Immediately before bougienage, you should refrain from eating or drinking liquids, and also perform toileting of the genitals.
Treatment
In most cases, treatment of urethritis does not require hospitalization. Therapy is carried out at home with medications recommended by the doctor. To monitor the dynamics of treatment, it is necessary to periodically visit the clinic during the treatment process.
If a bacterial infection is confirmed, therapy is carried out using antibacterial drugs. These can be pills, intramuscular or intravenous infections. Women can also use vaginal suppositories. When treating men, instillations may be prescribed. During the procedures, a medicinal substance is infused through a catheter into the urethra.
With proper and rapid therapy using highly effective medications, which can now be purchased inexpensively in pharmacies, a positive result is guaranteed in the shortest possible time. In the absence of correct timely treatment, urethritis quickly becomes chronic.
HOW IS SPERM FORMED?
The production of sperm is called spermatogenesis. It can only occur under the influence of hormones, therefore, until puberty (12–16 years), the boy’s body is not able to produce sperm.
When testosterone levels rise in a teenager's body, spermatogonia, special stem cells in the testicles, are activated. They turn into spermatocytes. These cells contain a double set of chromosomes; after division, secondary spermatocytes are formed, each containing one set of chromosomes.
A person has two sex chromosomes - X and Y. An egg can only contain chromosome X. A sperm can contain either X or Y. If the egg is fertilized by a sperm with a Y chromosome, the result is a boy, if with an X, the result is a girl.
Spermatid cells are formed from spermatocytes. The complex process of sperm formation does not end there. Spermatid cells go through a process known as spermiogenesis. They grow tails and acquire features characteristic of sperm. After maturation in the epididymis, they are ready to leave the man’s body in search of an egg.
A mature sperm can move at a speed of 20 cm per hour. And this despite the fact that its length is 0.05 mm.