The relevance of studying late-life depression is primarily due to the high frequency of this pathology in older age groups of the population. According to various authors [1–3], 10 to 25% of elderly people suffer from depression, and depression in older people is often not recognized by doctors, which leads to prolongation and chronicity of the disease [1, 3, 4].
Most researchers agree that the prevalence rates of depressive disorders among older age groups are almost 2 times higher than the corresponding estimates for younger populations. Depression increases the risk of hospitalization in older people, leads to an increase in the length of their hospital stay for somatic diseases, causes a worse therapeutic response to medications, is one of the most important causes of suicide, and increases the risk of developing dementia. According to WHO experts, by 2022 depression will become the leading disease in terms of costs of care for patients in older age groups of the population. If we take into account that at a later age there is an increased risk of unwanted side effects and complications of psychopharmacotherapy associated with age-related characteristics of the processes of biotransformation and elimination of drugs, as well as the burden of elderly patients with comorbid somatic and neurological diseases requiring constant treatment, then this makes it understandable why treatment of late-life depression is a particularly difficult problem in modern psychiatry. In this regard, increasing the effectiveness and safety of psychopharmacotherapy in the treatment of elderly and senile patients with depressive disorders becomes highly relevant.
In recent years, it has been established that the pathogenetic mechanisms of depression are associated not only with disorders of monoaminergic synaptic transmission and dysfunction of neuroendocrine systems, but also with disorders of the circadian system, characterized by disorganization of the endogenous (internal) rhythms of the body [5]. To date, one of the concepts of the pathogenesis of depressive disorders is chronobiological, based on the fact that depressive states are often accompanied by desynchronization of biological rhythms, primarily circadian (circadian), corresponding to daily fluctuations, i.e., a 24-hour period.
Disturbances in circadian rhythms can alter a variety of biological processes, such as the sleep-wake cycle, hormone release, body temperature (flattening diurnal temperature fluctuations), and other important physical functions. A study of pathophysiological indicators in depression, reflecting disturbances in the sleep-wake circadian rhythm, showed the presence of a number of biochemical abnormalities. Among them are a decrease in the nighttime concentration of melatonin in the blood plasma, a violation of the periodicity of fluctuations in the concentrations of prolactin, cortisol, thyroid-stimulating hormone, etc. [6].
Within the chronobiological concept of depression, a large place is given to sleep disorders. Some researchers consider early detection and treatment of sleep disorders as a way to prevent recurrent depression [7], as well as predict relapse of the disease [8]. This is confirmed by data from epidemiological studies indicating that more than 80% of adults suffering from depression have sleep disorders [9]. In older adults, insomnia is the most common complaint [10], and its frequency increases with increasing age [11].
Some authors [12] consider circadian rhythm disturbances and depressive disorders as two interrelated changes characteristic of old age. Restoration of circadian rhythms leads to normalization of sleep structure: in patients with depression, the duration of the slow-wave sleep phase increases without changing its total duration and the number of REM sleep phases, the severity of depressive symptoms changes during the day, which leads to the leveling of affective disorder [13]. In this regard, new antidepressants that are effective against insomnia and do not cause daytime sleepiness may represent a treatment for late-life depression. Such antidepressants include Valdoxan [14]. The first experience of using this drug in the treatment of depressive disorders in outpatient gerontopsychiatric practice turned out to be very positive [15].
Valdoxan is an agonist of MT1 and MT2 melatonin receptors and an antagonist of 5-HT2C serotonin receptors [16]. At the same time, Valdoxan does not show significant affinity for histaminergic, beta- and alpha-adrenergic, cholinergic, benzodiazepine and dopaminergic receptors, and does not affect the uptake of monoamines. Restoring the synchronization of circadian rhythms when taking Valdoxan is carried out by stimulating the receptors of melatonin, a hormone that plays a major role in synchronizing the body's circadian rhythms, including the sleep-wake cycle. According to V. Audinot et al. [16], the drug does not have a negative effect on memory and attention and has no behavioral toxicity. The use of Valdoxan has no effect on blood pressure and heart rate, and does not cause addiction or withdrawal syndromes (even with abrupt withdrawal).
The purpose of this study was to evaluate the therapeutic efficacy, tolerability and safety of Valdoxan in the treatment of depression in elderly psychiatric patients.
Material and methods
The study included patients aged 60 years and older with depression of varying severity who were treated in the geriatric psychiatric clinic of the Scientific Center for Mental Health.
The patients were prescribed Valdoxan in standard doses of 25-50 mg per day for 42 days. Valdoxan (Valdoxan) was prescribed in accordance with the instructions 1 time per day at the same time (evening) at a dose of 25 mg (1 tablet) in the first 2 weeks of therapy. If necessary, the daily dose was increased to a maximum of 50 mg 1 time (in the evening).
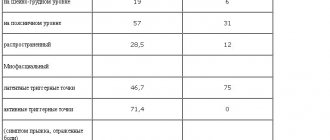
The study included 20 patients, 6 (30%) men and 14 (70%) women aged 60 to 79 years (average - 69.9±6.06 years) (Table 1).
Table 1. Demographic and clinical characteristics of the group of patients treated with Valdoxan
All patients were diagnosed with a major depressive episode (DE) in accordance with the DSM-IV-TR or ICD-10 classification, including 2 patients with a single episode of DE, 13 with a depressive phase within the framework of recurrent depressive disorder (RDD), and 5 - within the framework of bipolar affective disorder (BD). The patients had no history of other mental disorders or primary organic diseases.
According to ICD-10 criteria, depression corresponded to mild DE in 6 (30%) patients, moderate DE in 13 (65%) patients, and severe DE in 1 (5%) (see Table 1).
All patients underwent a full clinical examination (psychopathological, psychometric, therapeutic and neurological).
To assess the therapeutic effectiveness of treatment, standardized rating scales were used: the Hospital Anxiety and Depression Scale (HADS), the Snight-Hamilton Anhedonia Rating Scale (SHAPS), and the Clinical Global Impression (CGI) scale. The patients' condition was assessed on these scales before the start of treatment (day 0), on the 7th, 14th, 28th and 42nd days of therapy. To assess the quality of life of patients, a special questionnaire SF-36 was used. The Mini Mental State Examination (MMSE) was used to assess cognitive functioning. These questionnaires were assessed before and after the end of treatment (on the 42nd day of therapy).
The effectiveness of therapy was determined by the degree of reduction in the average scores for the group of patients on the SHAPS, HADS, CGI-S and CGI-I scales on the 7th, 14th, 28th and 42nd days of treatment in relation to the initial assessment (in %).
Adverse effects of therapy were assessed using the UKU drug side effect scale before the start, on the 7th and 42nd days of treatment.
The HADS consists of 14 items and has 2 subscales (to assess levels of anxiety and depression). When assessing the severity of anxiety and depression, the total score for each subscale was taken into account. The total score from 0 to 7 corresponds to the norm, 8-10 points - subclinical level, 11 points and above - clinically expressed anxiety and depression.
The CGI scale used a graded assessment of the severity of the condition (CGI-S subscale), where 1 point corresponded to the fact that the patient is not sick, 2 points - borderline state (subclinical level), 3 points - mild, 4 points - moderate, 5 points - moderate-severe condition, 6 points - severe condition, 7 points - extremely severe condition. The CGI-I subscale assessed the dynamics of the state during therapy compared to its initial level: 1 point corresponded to pronounced, 2 points to moderate and 3 points to minimal improvement; 4 points corresponded to no change, 5 points to minimal deterioration, 6 points to deterioration, and 7 points to marked deterioration.
The SF-36 questionnaire is used to assess the quality of life of patients. It consists of 36 items, which are grouped into 8 scales: physical functioning, role functioning, bodily pain, general health, vitality, social functioning, emotional state and mental health. The results are scored on each of 8 scales, which are designed in such a way that a higher score indicates a better level of quality of life. The following indicators are quantified: 1. Physical functioning (PF), reflecting the degree to which physical condition limits the performance of physical activities (self-care, walking, climbing stairs, carrying heavy objects, etc.). Low scores on this scale indicate that the patient's physical activity is significantly limited by his health status. 2. Role functioning due to physical condition (RP) - the influence of physical condition on daily role activities (work, daily duties). Low scores on this scale indicate that daily activities are significantly limited by the patient's physical condition. 3. Pain intensity (BP) and its impact on the ability to perform daily activities. Low scores on this scale indicate that pain significantly limits the patient's activity. 4. General health (GH) - the patient’s assessment of his or her health status. 5. Vital activity (VT) implies feeling full of strength and energy or, on the contrary, exhausted. Low scores indicate patient fatigue and decreased vital activity. 6. Social functioning (SF), determined by the degree to which physical or emotional conditions limit the social activities of patients. Low scores indicate a significant limitation of social contacts, a decrease in the level of communication due to deterioration of physical and emotional condition. 7. Role functioning due to emotional state (RE) involves assessing the extent to which the emotional state interferes with the performance of work or other daily activities (including more time spent, less work, less quality work, etc.). Low scores on this scale are interpreted as a limitation in the performance of daily work due to a deterioration in the emotional state. 8. Mental health (MH) characterizes mood, the presence of depression, anxiety, and a general indicator of positive emotions. Low scores indicate the presence of depression, anxiety, and mental ill-being. All scales are grouped into two indicators: “physical health component” (Physical health - PH) and “psychological health component” (Mental Health - MH).
The level of cognitive activity was assessed using the MMSE scale, according to which a total score of 28 points and above corresponds to the norm; a score from 25 to 27 points indicates mild cognitive impairment; 24 points and below indicate cognitive deficit in the degree of dementia.
At the beginning of therapy, the anxiety component of depression according to HADS was on average 13.0±4.13 points for the group, the depressive component was 13.58±3.44 points, the average total score of anxiety and depression was 26.05±6.29 points, and the average anhedonia level score (on the SHAPS scale) was 8.8±3.12 points. The level of cognitive activity of most patients corresponded to the age norm with an average group score on the MMSE scale of 26.76±2.30 points (Table 2).
Table 2. Dynamics of average group indicators (in points) on rating scales during therapy with Valdoxan
In the study group, a high burden of patients with concomitant chronic somatic pathology was established. The total number of chronic somatic diseases averaged 3.85±1.42 per patient. The most common diagnoses in the studied cohort of patients were cerebrovascular (90%) and cardiovascular (50%) pathology, as well as diabetes mellitus (25%).
Statistical analysis of the results was carried out using the Statistica 6.0 for Windows program using nonparametric methods (paired Wilcoxon test). To describe quantitative characteristics, average values and standard deviations were used. Differences were considered significant at p
<0,05.
results
18 of 20 patients completed the complete course of therapy with Valdoxan; 2 patients were prematurely excluded from the therapeutic program: in one case, on the 14th day of therapy due to a deterioration in the patient’s mental state (increased symptoms of depression with the addition of hypochondriacal delusions), in another case, also on the 14th day of therapy due to the development of undesirable phenomena.
The majority of patients (14 people - 78%) from the 3rd week of therapy received Valdoxan at a dose of 50 mg per day, and 4 (22%) patients continued treatment at an initial dose of 25 mg per day.
A significant reduction in total indicators of anxiety and depression on the HADS scale was observed after just 1 week of therapy (see Table 2). By this time, the average total score on the scale decreased from 26.05 to 22.52 ( p
<0.01), the average improvement rate was 13.14%.
By the 14th day of therapy, the average total score continued to decrease and amounted to 16.88 ( p
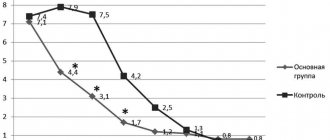
= 0.0006), the average degree of improvement was 34.46%. On the 28th day of therapy, this indicator was 14.58 points, the average degree of improvement was 43.46%. By the end of the therapeutic course (on the 42nd day of therapy), the average total score on the HADS scale decreased to 10.0, and the average degree of improvement in the group reached 61.61% (see Table 2, Fig. 1).
Rice.
1. Dynamics of group average total indicators (in points) on the HADS scale during therapy with Valdoxan in elderly patients with depression. Here and in Fig. 2: differences compared to baseline: * - p<0.01 (paired Wilcoxon test). The level of anxiety disorders, assessed on the HADS scale, at the beginning of therapy was 13.0 points and significantly decreased throughout the therapeutic course of Valdoxan: after 1 week the anxiety level was 10.17 points, by the 14th day of therapy - 7.41, by the 28th - 6.70, and by the end of therapy it had decreased to 4.41 points (see Table 2, Fig. 1).
The level of depression on the HADS scale before treatment was 13.58 points, it progressively decreased with each subsequent visit: by the 7th day of therapy - to 12.23 points, by the 14th - to 9.41, by the 28th - up to 7.88 and by the 42nd day - up to 5.58. In the group as a whole, the depressive component was reduced by 58.91% compared to the initial level. As one would expect, the dynamics of reduction in the anxiety component of depression were somewhat faster than the reduction in depressive disorders themselves. These differences leveled out by the end of the 1st month of therapy (see Table 2, Fig. 1).
Anhedonia is considered one of the leading manifestations of depression and is often considered as its potential constitutional marker. Anhedonia is understood as dissatisfaction with the incompleteness of mental functions, the impossibility of self-expression, the bitterness of existence, depriving the patient of the opportunity to receive pleasure and positive emotions. Therefore, the effective therapeutic effect of an antidepressant on the manifestations of anhedonia can be considered as an important component of the success of depression treatment. During therapy with Valdoxan, positive dynamics in the form of a reduction in anhedonia were noted already in the early stages of treatment, with a subsequent increase in the positive effect. According to the SHAPS anhedonia rating scale, a statistically significant reduction in the manifestations of anhedonia was noted after 2 weeks of treatment. By this time, anhedonia was reduced by 31.57% and subsequently continued to decrease: by the 28th day - by 52.11% and by the end of therapy - by 69.29% (see Table 2, Fig. 2).
Rice. 2. Dynamics of the group average total index of anhedonia (in points) on the SHAPS scale during therapy with Valdoxan in elderly patients with depression.
After completion of therapy with Valdoxan, the quality of life of patients also improved. Analysis of the dynamics of quality of life indicators, carried out using the SF-36 questionnaire, showed an improvement in all assessed parameters compared to the baseline. Indicators of both physical and psychological components of health improved significantly (Table 3). The greatest improvements concerned indicators such as life and social activity and mental health (Fig. 3).
Table 3. Dynamics of group average indicators of quality of life according to the SF-36 questionnaire in patients treated with Valdoxan
Rice. 3. The effectiveness of therapy in terms of quality of life according to the SF-36 questionnaire in patients treated with Valdoxan.
One of the features of depression in old age is the frequent presence of cognitive disorders of varying severity. Therefore, choosing a drug that does not have a negative effect on cognitive functioning is one of the most important tasks of geriatric psychopharmacotherapy. A study of the effect of Valdoxan on the cognitive functioning of patients, carried out using the MMSE test, showed not only the absence of deterioration, but also some improvement in this indicator after the end of course therapy. The average initial group average score on the MMSE scale increased by the end of treatment from 26.76±3.30 to 28.17±2.0 points, i.e., improved by 5.27% (see Table 2). This effect of the drug is probably associated with a reduction in the anxiety component and impaired concentration associated with depression.
When assessing the effectiveness of Valdoxan therapy on the CGI scale, rapid positive dynamics were noted. Already by the 14th day of therapy, improvement of varying degrees of severity was noted in 55% of patients, in 35% the condition remained without significant dynamics, and in 2 (10%) patients the condition worsened (Table 4). On the 28th day of therapy, an improvement in mental state on the CGI scale was observed in 88.9% of patients, and by the end of therapy - in 94.5% (Fig. 4). In general, the results of the analysis of tolerability of therapy with Valdoxan showed good tolerability of therapy. Adverse events that occurred in 2 patients were mild or moderate. In 1 patient, on the 14th day of therapy, there was a deterioration in the condition, which was manifested by worsening symptoms of depression with the addition of hypochondriacal delusions, and therefore the patient was excluded from this therapeutic program. In the second case, the deterioration of the condition was associated with the development of an adverse event in the form of an allergic reaction, which also led to early discontinuation of the drug.
Table 4. Distribution of patients with varying degrees of change in condition according to the CGI scale during therapy with Valdoxan in relation to the total number of patients
Rice. 4. The proportion of patients with varying degrees of change in CGI scale indicators (in % of the total number of patients) during therapy with Valdoxan.
Valdoxan
Pharmacodynamics
Antidepressant, agonist of melatonin MT1 and MT2 receptors and antagonist of serotonin 5-HT2c receptors.
Agomelatine is active in validated models of depression (learned helplessness test, despair test, moderate chronic stress), as well as in models with desynchronization of circadian rhythms, as well as in experimental situations of anxiety and stress. It has been shown that agomelatine does not affect the uptake of monoamines and has no affinity for α-, β-adrenergic receptors, histaminergic receptors, cholinergic, dopaminergic and benzodiazepine receptors.
Agomelatine enhances the release of dopamine and norepinephrine, especially in the prefrontal cortex and does not affect the concentration of extracellular serotonin. In experimental studies in animals with circadian rhythm desynchronization, agomelatine was shown to restore circadian rhythm synchronization through stimulation of melatonin receptors.
Agomelatine helps restore normal sleep structure, reduce body temperature and release melatonin.
The effectiveness of short-term use of agomelatine (therapy for 6-8 weeks) in doses of 25-50 mg in patients with major depressive episodes has been shown.
Agomelatine has also been shown to be effective in patients with more severe forms of depressive disorder (Hamilton scale score ≥25).
Agomelatine was also effective for initially high levels of anxiety, as well as for combined anxiety and depressive disorders.
The supporting antidepressant effect of agomelatine was confirmed (with a study duration of 6 months) at a dose of 25-50 mg 1 time/day. The results of the study confirmed the anti-relapse effectiveness of agomelatine, which was assessed by the time until the onset of disease relapse (p=0.0001). The relapse rate in the group of patients taking agomelatine was 22%, in the placebo group - 47%.
Agomelatine was shown to be effective in 6 of 7 clinical studies (benefit (2 studies) or comparable efficacy (4 studies)) in heterogeneous populations of adult patients with depression compared with selective serotonin reuptake inhibitors (SSRIs)/selective norepinephrine reuptake inhibitors (SNRIs). ) (sertraline, escitalopram, fluoxetine, venlafaxine or duloxetine). Antidepressant effect was assessed using the Hamilton scale (17-item version) as either a primary or secondary endpoint.
Agomelatine does not have a negative effect on alertness and memory; in patients with depression, agomelatine at a dose of 25 mg increases the duration of the slow-wave sleep phase without changing the number and duration of REM sleep phases. Taking agomelatine at a dose of 25 mg also promotes a faster onset of sleep with a decrease in heart rate and improved sleep quality (starting from the first week of treatment); however, there is no inhibition during the daytime.
When taking agomelatine, there was a tendency to reduce the frequency of sexual dysfunction (impact on arousal and orgasm).
Taking agomelatine has no effect on heart rate and blood pressure, does not cause sexual dysfunction, does not cause withdrawal syndrome (even with abrupt cessation of treatment) and addiction syndrome.
The effectiveness of agomelatine at a dose of 25-50 mg 1 time / day was confirmed in elderly patients (younger than 75 years) with depression during an 8-week clinical trial. There is no evidence of a significant effect in patients aged 75 years and older. The tolerability of agomelatine in elderly patients is comparable to that in young patients.
In a 3-week controlled study of patients with major depressive disorder who were not responding to paroxetine (SSRI) or venlafaxine (SNRI), withdrawal syndrome was observed when switching from these antidepressants to agomelatine. Withdrawal syndrome appeared both after immediate cessation of treatment with previously prescribed SSRIs/SNRIs, and during their gradual withdrawal, which could be mistakenly taken as a manifestation of the low effectiveness of agomelatine at the initial stage of treatment.
The number of patients experiencing at least one withdrawal symptom 1 week after SSRI/SNRI discontinuation was lower in the long-dose taper group (gradually tapering the SSRI/SNRI dose over 2 weeks) than in the rapid taper group. dose (gradual reduction of SSRI/SNRI dose over 1 week), and than with immediate withdrawal: 56.1%, 62.6% and 79.8% of patients, respectively.
Pharmacokinetics
Suction
After oral administration, agomelatine is rapidly (≥80%) absorbed from the gastrointestinal tract. Cmax in plasma is achieved 1-2 hours after administration. Absolute bioavailability after taking a therapeutic dose is low (<5%); interindividual variability is significant. Bioavailability is higher in women than in men. Bioavailability increases with oral contraceptives and decreases with smoking.
When the drug was prescribed in therapeutic doses, Cmax increased in proportion to the dose. When taken in higher doses, a more pronounced “first pass” effect through the liver was observed. Meal intake (both regular and high-fat) did not affect either bioavailability or extent of absorption. Interindividual variability increased when eating a high-fat diet.
Distribution
Vd in the equilibrium state was about 35 l. Plasma protein binding is 95%, regardless of drug concentration, age or the presence of renal failure.
In case of liver failure, a twofold increase in the free fraction of the drug was observed.
Metabolism
After oral administration, agomelatine undergoes rapid oxidation, mainly due to CYP1A2 and CYP2C9. The CYP2C19 isoenzyme is also involved in the metabolism of agomelatine, but its role is less significant.
The main metabolites in the form of hydroxylated and demethylated agomelatine are inactive, quickly bind and are excreted by the kidneys.
Removal
T1/2 from plasma is from 1 to 2 hours. Elimination occurs quickly. Metabolic clearance is about 1100 ml/min. Excretion occurs mainly by the kidneys (80%) in the form of metabolites. The amount of unchanged drug in the urine is insignificant. With repeated administration of the drug, the kinetics do not change.
Pharmacokinetics in special clinical situations
In patients with severe renal failure, a single dose of agomelatine at a dose of 25 mg did not significantly change the pharmacokinetic parameters. Due to limited clinical experience, caution should be exercised when prescribing agomelatine to patients with moderate to severe renal impairment.
When agomelatine was prescribed at a dose of 25 mg to patients with mild (Child-Pugh class A) and moderate (Child-Pugh class B) chronic liver failure against the background of liver cirrhosis, an increase in its plasma concentration by 70 and 140 times was noted , respectively, compared with volunteers matched for sex, age and smoking behavior, but without liver failure.
When agomelatine 25 mg was administered to elderly patients (65 years and older), it was noted that the mean AUC and mean Cmax were 4 times and 13 times higher, respectively, in patients aged 75 years and older compared with patients younger 75 years old. The total number of patients treated with the 50 mg dose was too low to draw any conclusions. No dose adjustment is required depending on age.
There are no data on racial differences in pharmacokinetic parameters.