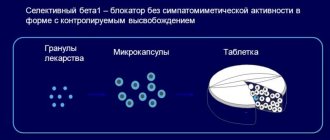
Betaloc ZOK
Betaloc® ZOK is intended for daily use once a day; it is recommended to take the drug in the morning. The Betaloc® ZOK tablet should be swallowed with liquid. Tablets (or halved tablets) should not be chewed or crushed. Food intake does not affect the bioavailability of the drug.
When selecting a dose, it is necessary to avoid the development of bradycardia
Arterial hypertension
50-100 mg 1 time/day. If necessary, the dose can be increased to 100 mg 1 time / day or Betaloc® ZOK can be used in combination with other antihypertensive agents, preferably a diuretic and a calcium channel blocker dihydropyridine derivative.
Angina pectoris
100-200 mg Betaloc® ZOK 1 time/day. If necessary, another antianginal drug may be added to therapy.
Stable symptomatic chronic heart failure with impaired left ventricular systolic function
Patients must be in stable chronic heart failure without episodes of exacerbation during the last 6 weeks and without changes in primary therapy during the last 2 weeks.
Treatment of heart failure with beta-blockers can sometimes lead to a temporary worsening of the symptomatic picture. In some cases, it is possible to continue therapy or reduce the dose; in some cases, it may be necessary to discontinue the drug.
Stable chronic heart failure, functional class II
The recommended initial dose of Betaloc® ZOK for the first 2 weeks is 25 mg 1 time / day. After 2 weeks of therapy, the dose can be increased to 50 mg 1 time / day, and then can be doubled every 2 weeks.
Maintenance dose for long-term treatment: 200 mg Betaloc® ZOK 1 time/day.
Stable chronic heart failure, III-IV functional class
The recommended initial dose for the first 2 weeks is 12.5 mg Betaloc® ZOK (half a 25 mg tablet) 1 time/day. The dose is selected individually. During the period of increasing the dose, the patient should be monitored, because In some patients, heart failure symptoms may worsen.
After 1-2 weeks, the dose can be increased to 25 mg Betaloc® ZOK 1 time / day. Then, after 2 weeks, the dose can be increased to 50 mg 1 time / day. For patients who tolerate the drug well, the dose can be doubled every 2 weeks until a maximum dose of 200 mg Betaloc® ZOK is reached 1 time / day.
In case of arterial hypotension and/or bradycardia, it may be necessary to reduce concomitant therapy or reduce the dose of Betaloc® ZOK. Arterial hypotension at the beginning of therapy does not necessarily indicate that a given dose of Betaloc® ZOK will not be tolerated during further long-term treatment. However, the dose should not be increased until the condition has stabilized. Monitoring of renal function may be required.
Heart rhythm disturbances
100-200 mg Betaloc® ZOK 1 time/day.
Maintenance treatment after myocardial infarction
200 mg Betaloc® ZOK 1 time/day.
Functional cardiac disorders accompanied by tachycardia
100 mg Betaloc® ZOK 1 time / day, if necessary, the dose can be increased to 200 mg / day.
Preventing migraine attacks
100-200 mg Betaloc® ZOK 1 time/day.
Renal dysfunction
There is no need to adjust the dose in patients with impaired renal function.
Liver dysfunction
Usually, due to the low degree of binding to plasma proteins, no dose adjustment of metoprolol is required. However, in severely impaired liver function (in patients with severe cirrhosis or portocaval anastomosis), a dose reduction may be required.
Elderly age
There is no need to adjust the dose in elderly patients.
Children
Experience with the use of Betaloc® ZOK in children is limited.
Overdose
Metoprolol at a dose of 7.5 g in an adult caused intoxication with a fatal outcome. A 5-year-old child who took 100 mg of metoprolol showed no signs of intoxication after gastric lavage. Taking 450 mg of metoprolol by a 12-year-old teenager resulted in moderate intoxication. Taking 450 mg of metoprolol by a 12-year-old teenager resulted in moderate intoxication. Administration of 1.4 g and 2.5 g of metoprolol to adults caused moderate and severe intoxication, respectively. Taking 7.5 g by adults resulted in extremely severe intoxication.
Symptoms: with an overdose of metoprolol, the most serious symptoms are those from the cardiovascular system, but sometimes, especially in children and adolescents, symptoms from the central nervous system and suppression of pulmonary function, bradycardia, AV block I-III degree, asystole, marked decrease in Blood pressure, poor peripheral perfusion, heart failure, cardiogenic shock; depression of pulmonary function, apnea, as well as increased fatigue, impaired consciousness, loss of consciousness, tremor, convulsions, increased sweating, paresthesia, bronchospasm, nausea, vomiting, possible esophageal spasm, hypoglycemia (especially in children) or hyperglycemia, hyperkalemia; effects on the kidneys; transient myasthenic syndrome; concomitant use of alcohol, antihypertensive drugs, quinidine or barbiturates may worsen the patient's condition. The first signs of overdose can be observed 20 minutes to 2 hours after taking the drug.
Treatment: administration of activated carbon, gastric lavage if necessary. Important! Atropine (0.25-0.5 mg IV for adults, 10-20 mcg/kg for children) should be given before gastric lavage (due to the risk of vagus nerve stimulation). If necessary, maintain a patent airway (intubation) and provide adequate ventilation. Replenishment of circulating blood volume and glucose infusion. ECG monitoring. Atropine 1.0-2.0 mg IV, repeat administration if necessary (especially in case of vagal symptoms). In case of (suppression of) myocardial depression, infusion of dobutamine or dopamine is indicated. You can also use glucagon 50-150 mcg/kg IV at 1-minute intervals. In some cases, adding epinephrine to therapy may be effective. For arrhythmia and a widened ventricular (QRS) complex, sodium solutions (chloride or bicarbonate) are infused. It is possible to install an artificial pacemaker. Cardiac arrest due to an overdose may require resuscitation for several hours. Terbutaline (injected or inhaled) can be used to relieve bronchospasm. Symptomatic treatment is carried out.
Betaloc®
Co-administration of Betaloc ® with the following drugs should be avoided:
Barbituric acid derivatives: barbiturates (study conducted with phenofarbital) slightly increase the metabolism of metoprolol due to enzyme induction.
Propafenone: When propafenone was prescribed to four patients treated with metoprolol, an increase in plasma concentrations of metoprolol was observed by 2-5 times, while two patients experienced side effects characteristic of metoprolol. This interaction was confirmed in a study on 8 volunteers. The interaction is likely due to propafenone's inhibition, like quinidine, of the metabolism of metoprolol via the cytochrome P4502D6 system. Taking into account the fact that propafenone has the properties of a beta-blocker, the joint administration of metoprolol and propafenone does not seem appropriate.
Verapamil: The combination of beta-blockers (atenolol, propranolol and pindolol) and verapamil can cause bradycardia and lead to a decrease in blood pressure. Verapamil and β-blockers have a complementary inhibitory effect on atrioventricular conduction and sinus node function.
The combination of Betaloc® with the following drugs may require dose adjustment:
Class I Antiarrhythmics: Class I antiarrhythmics and β-blockers may result in additive negative inotropic effects, which can lead to serious hemodynamic side effects in patients with impaired left ventricular function. This combination should also be avoided in patients with sick sinus syndrome and impaired AV conduction. The interaction is described using disopyramide as an example.
Amiodarone: Concomitant use of amiodarone and metoprolol may result in severe sinus bradycardia. Given the extremely long half-life of amiodarone (50 days), a possible interaction should be considered long after discontinuation of amiodarone.
Diltiazem: Diltiazem and β-blockers mutually enhance the inhibitory effect on AV conduction and sinus node function. When metoprolol was combined with diltiazem, cases of severe bradycardia were observed.
Nonsteroidal anti-inflammatory drugs (NSAIDs): NSAIDs reduce the antihypertensive effect of beta-blockers. This interaction is best documented for indomethacin. There is no reported interaction observed for sulindac. In studies with diclofenac, the described reaction was not observed.
Diphenhydramine: Diphenhydramine reduces the clearance of metoprolol to α-hydroxymetoprolol by 2.5 times. At the same time, an increase in the effect of metoprolol is observed.
Epinephrine (adrenaline): 10 cases of severe hypertension and bradycardia have been reported in patients taking non-selective beta-blockers (including pindolol and propranolol) and receiving epinephrine (adrenaline). The interaction was also observed in the group of healthy volunteers. It is assumed that similar reactions can be observed when epinephrine is used together with local anesthetics if it accidentally enters the vascular bed. It is assumed that this risk is much lower with the use of cardioselective beta-blockers.
Phenylpropanolamine: Phenylpropanolamine (norephedrine) in a single dose of 50 mg can cause an increase in diastolic blood pressure to pathological values in healthy volunteers. Propranolol mainly prevents the increase in blood pressure caused by phenylpropanolamine. However, β-blockers may cause paradoxical hypertension reactions in patients receiving high doses of phenylpropanolamine. Several cases of hypertensive crisis have been reported while taking phenylpropanolamine.
Quinidine: Quinidine inhibits the metabolism of metoprolol in a special group of patients with rapid hydroxylation (in Sweden, approximately 90% of the population), causing mainly a significant increase in plasma concentrations of metoprolol and increased beta-blockade. It is believed that a similar interaction is typical for other β-blockers in the metabolism of which cytochrome P4502D6 is involved.
Clonidine: Hypertensive reactions during abrupt withdrawal of clonidine may be exacerbated by concomitant use of beta-blockers. When used together, if clonidine is discontinued, discontinuation of β-blockers should begin several days before discontinuation of clonidine.
Rifampicin: Rifampicin may increase the metabolism of metoprolol, reducing plasma concentrations of metoprolol.
The concentration of metoprolol in the blood plasma may increase when combined with cimetidine, hydralazine, selective serotonin inhibitors such as paroxetine, fluoxetine and sertraline.
Patients concomitantly taking metoprolol and other β-blockers (eye drops) or monoamine oxidase inhibitors (MAOIs) should be closely monitored.
When taking β-blockers, inhalational anesthetics enhance the cardiodepressive effect.
While taking β-blockers, patients receiving oral hypoglycemic agents may require dose adjustment of the latter.
Cardiac glycosides, when used together with beta-blockers, can increase atrioventricular conduction time and cause bradycardia.