Pronoran 50 mg, 30 controlled-release film-coated tablets
Registration Certificate Holder
Les Laboratoires Servier (France)
Dosage form
Medicine – Pronoran® (Pronoran®)
Description
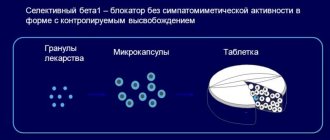
Controlled-release film-coated tablets
red, round, biconvex; Slight heterogeneity of coloring, degree of gloss and the presence of minor inclusions are allowed.
1 tab.
piribedil 50 mg
Excipients
: magnesium stearate - 5 mg, povidone - 20 mg, talc - 130 mg.
Shell:
carmellose sodium - 0.71 mg, polysorbate 80 - 0.30 mg, crimson dye [Ponceau 4R] - 3.87 mg, povidone - 6.31 mg, sodium bicarbonate - 0.15 mg, colloidal silicon dioxide - 0.27 mg, sucrose - 57.17 mg, talc - 50.37 mg, titanium dioxide - 0.78 mg, white beeswax - 0.07 mg.
15 pcs. - blisters (2) - cardboard packs with first opening control (if necessary). 29 pcs. - blisters (1) - cardboard packs with first opening control (if necessary). 30 pcs. - blisters (1) - cardboard packs with first opening control (if necessary).
Indications
- as an auxiliary symptomatic therapy for chronic impairment of cognitive function and neurosensory deficits during the aging process (disorders of attention, memory, etc.);
- Parkinson's disease in the form of monotherapy (in forms predominantly involving tremor) or as part of combination therapy with levodopa both in the initial and later stages of the disease, especially in forms including tremor;
- as an auxiliary symptomatic therapy for intermittent claudication resulting from obliterating diseases of the arteries of the lower extremities (stage 2 according to the Leriche and Fontaine classification);
- treatment of symptoms of ophthalmological diseases of ischemic origin (decreased visual acuity, narrowing of the visual field, decreased color contrast, etc.).
Contraindications for use
- increased individual sensitivity to piribedil and/or excipients included in the drug;
- collapse;
- acute stage of myocardial infarction;
- co-administration with antipsychotics (except clozapine) (see section “Drug interactions”);
- children under 18 years of age (due to lack of data).
With caution
Due to the fact that the drug contains sucrose, patients with intolerance to fructose, glucose or galactose, as well as patients with sucrose isomaltase deficiency (a rare metabolic disorder), are not recommended to take the drug.
pharmachologic effect
Pharmacodynamics
The active substance piribedil is a dopaminergic receptor agonist. Penetrates into the bloodstream of the brain, where it binds to dopaminergic receptors of the brain, showing high affinity and selectivity for dopaminergic receptors of types D2 and D3.
The mechanism of action of piribedil determines the main clinical properties of the drug for the treatment of Parkinson's disease both in the initial and later stages of the disease, affecting all major motor symptoms. In addition to its effect on dopaminergic receptors, Piribedil exhibits activity as an antagonist of two main α-adrenergic receptors of the central nervous system (type α2A and α2C).
The synergistic effect of piribedil, as an α2 receptor antagonist and dopaminergic receptor agonist of the brain, has been demonstrated in various animal models of Parkinson's disease: long-term use of piribedil leads to the development of less severe dyskinesia than the use of levodopa, with similar effectiveness in relation to the reversible akinesia associated Parkinson's disease.
Pharmacodynamic studies in humans have shown excitation of dopaminergic-type cortical electrogenesis both during awakening and during sleep, with clinical activity in relation to various functions controlled by dopamine. This activity has been demonstrated using behavioral or psychometric scales. In healthy volunteers, piribedil has been shown to improve attention and vigilance related to cognitive tasks.
The effectiveness of Pronoran® as monotherapy or in combination with levodopa in the treatment of Parkinson's disease was studied in three double-blind, placebo-controlled clinical studies (2 studies compared with placebo and 1 study compared with bromocriptine). The studies involved 1103 patients of stages 1-3 according to the Hoehn & Jahr scale, 543 of whom received Pronoran®. It has been shown that Pronoran® at a dosage of 150-300 mg/day is effective in affecting all motor symptoms with a 30% improvement in the Unified Parkinson's Disease Rating Scale (UPDRS) part III (motor) for more than 7 months with monotherapy and 12 months in combination with levodopa. Improvement in the activities of daily living portion of the UPDRS II was assessed in the same values.
In monotherapy, the statistically significant proportion of patients receiving rescue treatment with levodopa who received piribedil (16.6%) was lower than in the group of patients who received placebo (40.2%).
The presence of dopaminergic receptors in the vessels of the lower extremities explains the vasodilating effect of piribedil (increases blood flow in the vessels of the lower extremities).
Drug interactions
Due to the mutual antagonism between dopaminergic antiparkinsonian drugs and antipsychotics, simultaneous administration with antipsychotics (except clozapine) is contraindicated (see section "Contraindications").
Patients with extrapyramidal syndrome caused by taking antipsychotics should be treated with anticholinergic drugs and should not be prescribed dopaminergic antiparkinsonian drugs (due to the blocking of dopaminergic receptors by neuroleptics).
Dopaminergic receptor agonists may cause or worsen psychotic disorders. If the prescription of antipsychotics is required in patients with Parkinson's disease receiving treatment with dopaminergic antiparkinsonian drugs, the dose of the latter should be gradually reduced until permanent discontinuation (sudden withdrawal of dopaminergic drugs is associated with the risk of developing "neuroleptic malignant syndrome") (see section "Special instructions").
Antiemetic neuroleptics: Antiemetic drugs that do not cause extrapyramidal symptoms should be used.
Due to the mutual antagonism between dopaminergic antiparkinsonian drugs and tetrabenazine, coadministration of these drugs is not recommended.
It is not recommended to use piribedil together with alcohol.
Caution should be exercised when prescribing piribedil with other drugs that have a sedative effect.
Dosage regimen
Inside. The tablet should be taken after meals with half a glass of water without chewing.
For all indications (except Parkinson's disease)
the drug is prescribed in a dose of 50 mg (1 tablet) 1 time/day. In more severe cases - 50 mg 2 times a day.
For Parkinson's disease,
150-250 mg/day (3-5 tablets/day) is prescribed as monotherapy If it is necessary to take the drug at a dose of 250 mg, it is recommended to take 2 tablets. 50 mg morning and afternoon and 1 tab. In the evening.
When used in combination with levodopa drugs
The daily dose is 150 mg (3 tablets): it is recommended to divide into 3 doses.
When selecting a dose, if it is increased, it is recommended to titrate the dose, gradually increasing by 1 tablet. (50 mg) every 2 weeks.
Stopping treatment
Abrupt discontinuation of dopaminergic receptor agonist therapy is associated with a risk of developing neuroleptic malignant syndrome. To avoid this, the dose of piribedil should be reduced gradually until complete discontinuation.
Disorder of habits and urges
To avoid the risk of disorders of habits and desires, the lowest effective dose of the drug should be prescribed. If such symptoms occur, it is necessary to consider reducing the dose or gradually stopping drug therapy (see section "Special Instructions").
Patients with liver and/or kidney failure
There have been no studies of the use of piribedil in this group of patients.
In patients with hepatic and/or renal impairment, piribedil should be used with caution. Children and adolescents
The effectiveness and safety of piribedil in children and adolescents under 18 years of age have not been studied, and there are currently no data on the use of piribedil in this population. There are no valid indications for the use of piribedil in the pediatric population.
Overdose
Symptoms:
vomiting, which is caused by an effect on the chemoreceptor trigger zone; lability of blood pressure (increase or decrease); dysfunction of the gastrointestinal tract (nausea, vomiting).
Treatment:
drug withdrawal, symptomatic therapy.
Side effect
The reported adverse reactions when taking piribedil are dose-dependent and are mainly associated with its dopaminergic activity. They are moderate in nature, occur mainly at the beginning of treatment and disappear after discontinuation of the drug.
The frequency of side effects of piribedil is given in the following gradation: very often (≥1/10), often (≥1/100, <1/10), infrequently (≥1/1000, <1/100), rarely (≥1/100). 10,000, <1/1000), very rare (<1/10,000), unspecified frequency.
The following side effects may occur when taking the drug.
From the mental side:
often - mental disorders may occur, such as confusion, agitation, hallucinations (visual, auditory, mixed), which disappear when the drug is discontinued; unspecified frequency - aggression, psychotic disorders (delirium, delirium).
From the nervous system:
often - dizziness, which disappears when the drug is discontinued. Taking piribedil may be accompanied by drowsiness and, in extremely rare cases, severe drowsiness during the daytime, up to sudden falling asleep (see section “Special instructions”); unspecified frequency - dyskinesia (motor disorders).
From the cardiovascular system:
uncommon - hypotension, orthostatic hypotension with loss of consciousness or malaise or blood pressure lability.
From the gastrointestinal tract:
often - minor gastrointestinal disorders (nausea, vomiting, flatulence), which may subside, especially when selecting the appropriate individual dose. Dose selection by gradually increasing the dosage (50 mg every 2 weeks until the recommended dose is reached) leads to a significant reduction in the occurrence of these side effects.
Disorder of habits and desires:
In patients with Parkinson's disease treated with dopamine agonists, including piribedil, pathological gambling, increased libido and hypersexuality, compulsive shopping and overeating/compulsive eating have been reported (see section "Special Instructions").
General disorders and administration site disorders:
frequency unspecified - peripheral edema has been reported during dopamine agonist therapy.
Allergic reactions:
the risk of developing allergic reactions to the crimson dye included in the drug.
special instructions
Sudden falling asleep
In some patients (especially those with Parkinson's disease), while taking piribedil, a state of severe drowsiness sometimes suddenly occurs, even to the point of sudden falling asleep. Sudden falling asleep during daily activities, in some cases without awareness or without previous symptoms, is extremely rare, but nevertheless, patients driving a car and/or working on equipment requiring a high degree of attention should be warned about it. If such reactions occur, patients should refrain from driving and/or operating equipment that requires a high degree of attention. In addition, consideration should be given to reducing the dose of piribedil or discontinuing therapy with this drug.
Orthostatic hypotension
Dopamine agonists are known to disrupt systemic blood pressure regulation, which may result in orthostatic hypotension.
It is recommended to monitor blood pressure, especially at the beginning of treatment, due to the general risk of orthostatic hypotension associated with dopaminergic drugs.
Given the age of the population receiving piribedil therapy, the risk of falls, which may be caused by sudden sleep onset, hypotension or confusion, should be considered.
Disorder of habits and urges
Patients should be monitored for development of conduct disorder.
Patients and their caregivers should be warned about possible symptoms of habit disorder (compulsive gambling, increased libido and hypersexuality, compulsive shopping and overeating/compulsive eating) when taking dopamine agonists, incl. piribedila. If such symptoms occur, consider reducing the dose or gradually discontinuing drug therapy.
Behavioral disorders
Cases of conduct disorder have been reported and have been associated with symptoms such as confusion, agitation, and aggression. If such symptoms occur, consider reducing the dose or gradually discontinuing drug therapy.
Psychotic disorders
Dopamine agonists may cause or worsen psychotic disorders such as delirium, delirium and hallucinations (see section "Drug Interactions"). If such symptoms occur, consider reducing the dose or gradually discontinuing drug therapy.
Dyskinesia (motor disorders)
In patients with advanced Parkinson's disease while taking levodopa, dyskinesia may develop at the beginning of piribedil dose titration. In this case, the dose of piribedil should be reduced.
Neuroleptic malignant syndrome
Symptoms similar to neuroleptic malignant syndrome have been reported with abrupt discontinuation of dopaminergic drugs (see Dosage Regimen).
Peripheral edema
Peripheral edema has been reported during dopamine agonist therapy. This should be taken into account when prescribing piribedil.
Excipients
The crimson dye included in the drug increases the risk of allergic reactions in some patients.
Effects on the ability to drive vehicles and operate machinery
Patients who have had episodes of severe drowsiness and/or sudden falling asleep during piribedil therapy should refrain from driving vehicles and equipment requiring a high degree of attention until these reactions disappear.
Storage conditions
The drug should be stored out of the reach of children. Store at a temperature not exceeding 30°C.
Best before date
Shelf life: 3 years. Do not use after the expiration date stated on the package.
Use during pregnancy and breastfeeding
Restrictions during pregnancy - Contraindicated. Restrictions when breastfeeding - Contraindicated.
Fertility
Animal studies have not revealed direct or indirect negative effects on the development of the embryo and fetus, labor and postnatal development.
Pregnancy
In mice, piribedil has been shown to cross the placental barrier and distribute to fetal organs.
Due to the lack of data, the drug is not recommended for use during pregnancy and in women with preserved childbearing potential who do not use reliable contraceptive measures.
Breastfeeding period
Due to the lack of data, the drug is not recommended for use during breastfeeding.
Use in children
Restrictions for children - Contraindicated. Contraindicated in children and adolescents under 18 years of age.
Terms of sale
The drug is available with a doctor's prescription.
Contacts for inquiries
SERVIER JSC (Russia)
125196 Moscow st. Lesnaya, 7, fl. 7/8/9 BC “White Gardens” Tel. Fax
Compound
| Controlled-release film-coated tablets | 1 table |
| active substance: | |
| piribedil | 50 mg |
| excipients: magnesium stearate - 5 mg; povidone - 20 mg; talc - 130 mg | |
| shell: carmellose sodium -0.71 mg; polysorbate 80 -0.3 mg; crimson dye Ponceau 4R - 3.87 mg; povidone - 6.31 mg; sodium bicarbonate -0.15 mg; colloidal silicon dioxide -0.27 mg; sucrose - 57.17 mg; talc - 50.37 mg; titanium dioxide - 0.78 mg; white beeswax - 0.07 mg |
Interaction
Due to the mutual antagonism between dopaminergic antiparkinsonian drugs and antipsychotics, simultaneous administration with antipsychotics (except clozapine) is contraindicated.
1. Patients with extrapyramidal syndrome caused by taking antipsychotics should be prescribed therapy with anticholinergic drugs and should not be prescribed dopaminergic antiparkinsonian drugs (due to blocking of dopaminergic receptors by neuroleptics).
2. Dopaminergic antiparkinsonian drugs may cause or worsen psychotic disorders. If the prescription of antipsychotics is required in patients with Parkinson's disease receiving treatment with dopaminergic antiparkinsonian drugs, the dose of the latter should be gradually reduced until permanent discontinuation (sudden withdrawal of dopaminergic drugs is associated with the risk of developing neuroleptic malignant syndrome).
3. Antiemetic neuroleptics (antiemetic drugs that do not cause extrapyramidal symptoms should be used).
Due to the mutual antagonism between dopaminergic antiparkinsonian drugs and tetrabenazine, coadministration of these drugs is not recommended.
It is not recommended to use piribedil together with alcohol.
Caution should be exercised when prescribing piribedil with other drugs that have a sedative effect.
special instructions
In some patients (especially patients with Parkinson's disease), while taking piribedil, sometimes a sudden state of severe drowsiness occurs, even to the point of sudden falling asleep. This phenomenon is extremely rare, but nevertheless, patients driving a car and/or working on equipment that requires a high degree of attention should be warned about this. If such reactions occur, consider reducing the dose of piribedil or discontinuing therapy with this drug.
Given the age of the population receiving piribedil therapy, the risk of falls, which may be caused by sudden sleep onset, hypotension or confusion, should be considered.
Patients and their caregivers should be warned about possible symptoms of conduct disorder (compulsive gambling, increased libido and hypersexuality, compulsive shopping and binge eating disorder) while taking the drug. If such symptoms occur, consider reducing the dose or gradually discontinuing drug therapy.
The crimson dye included in the drug increases the risk of developing an allergic reaction in some patients.
Impact on the ability to drive vehicles and machinery. Patients who have experienced episodes of severe drowsiness and/or sudden falling asleep during piribedil therapy should refrain from driving vehicles or equipment requiring a high degree of alertness until these reactions resolve.
Side effects
The reported adverse reactions when taking piribedil are dose-dependent and are mainly associated with its dopaminergic activity. They are moderate in nature, occur mainly at the beginning of treatment and disappear after discontinuation of the drug.
The following side effects may occur when taking the drug:
From the gastrointestinal tract: often (≥1/100, <1/10) - minor gastrointestinal symptoms (nausea, vomiting, flatulence), these adverse reactions are reversible when the appropriate individual dose is selected. Dose selection by gradually increasing the dosage (50 mg every 2 weeks until the recommended dose is reached) leads to a significant reduction in the occurrence of these side effects.
From the side of the central nervous system: often (≥1/100, <1/10) - mental disorders may occur, such as confusion, hallucinations, agitation or dizziness, which disappear when the drug is discontinued.
Taking piribedil is accompanied by drowsiness and, in extremely rare cases, may be accompanied by severe drowsiness during the daytime, up to sudden falling asleep.
From the cardiovascular system: uncommon (≥1/1000, <1/100) - hypotension, orthostatic hypotension with loss of consciousness or malaise, or blood pressure lability.
Allergic reactions: the risk of developing allergic reactions to the crimson dye included in the drug.
Patients with Parkinson's disease treated with dopamine agonists, including piribedil, have reported gambling, increased libido and hypersexuality, compulsive shopping and binge eating.
Pharmacokinetics
Piribedil is quickly and almost completely absorbed from the gastrointestinal tract and distributed intensively.
Cmax of piribedil in blood plasma is achieved 3-6 hours after oral administration of the controlled-release dosage form. Plasma protein binding is average (the unbound fraction is 20–30%). Due to the low binding of piribedil to plasma proteins, the risk of drug interactions when used with other drugs is low.
Plasma elimination of piribedil is biphasic and consists of an initial phase and a second slower phase, leading to the maintenance of a steady-state plasma concentration of piribedil for more than 24 hours.
In a combined pharmacokinetic analysis, it was shown that T1/2 of piribedil after intravenous administration averages 12 hours and does not depend on the dose administered.
Piribedil is extensively metabolized in the liver and is excreted mainly in the urine: 75% of absorbed piribedil is excreted by the kidneys in the form of metabolites.
Pharmacodynamics
The active substance piribedil is a dopaminergic receptor agonist. Penetrates into the bloodstream of the brain, where it binds to dopaminergic receptors of the brain, showing high affinity and selectivity for dopaminergic receptors of types D2 and D3. The mechanism of action of piribedil determines the main clinical properties of the drug for the treatment of Parkinson's disease both in the initial and later stages of the disease, affecting all major motor symptoms. In addition to its effect on dopaminergic receptors, Piribedil exhibits activity as an antagonist of two main α-adrenergic receptors of the central nervous system (type α2A and α2C). The synergistic effect of piribedil as an α2 receptor antagonist and dopaminergic receptor agonist of the brain has been demonstrated in various animal models of Parkinson's disease: long-term use of piribedil leads to the development of less severe dyskinesia than the use of levodopa, with similar effectiveness in relation to the reversible akinesia associated with the disease Parkinson's.
Pharmacodynamic studies in humans have shown excitation of dopaminergic-type cortical electrogenesis, both upon awakening and during sleep, with clinical activity in various dopamine-controlled functions, this activity has been demonstrated using behavioral or psychometric scales. In healthy volunteers, piribedil has been shown to improve attention and vigilance related to cognitive tasks.
The effectiveness of Pronoran® as monotherapy or in combination with levodopa in the treatment of Parkinson's disease was studied in three double-blind, placebo-controlled clinical trials (2 studies compared with placebo and one compared with bromocriptine). The studies involved 1103 patients of stages 1–3 according to the Hoehn & Jahr scale, 543 of whom received Pronoran®.
It has been shown that Pronoran® at a dosage of 150–300 mg/day is effective in affecting all motor symptoms with a 30% improvement on the Unified Parkinson's Disease Rating Scale (UPDRS, part III - motor) for more than 7 months with monotherapy and 12 months in combination with levodopa. Improvement in part II of the UPDRS scale - activities of daily living - was assessed in the same values.
In monotherapy, the statistically significant proportion of patients requiring rescue treatment with levodopa who received piribedil (16.6%) was lower than that in the placebo group (40.2%).
The presence of dopaminergic receptors in the vessels of the lower extremities explains the vasodilating effect of piribedil (increases blood flow in the vessels of the lower extremities).
Directions for use and doses
Inside, after eating, without chewing, with 1/2 glass of water.
For all indications except Parkinson's disease - 50 mg (1 tablet) 1 time per day. In more severe cases - 50 mg 2 times a day.
Parkinson's disease: monotherapy - from 150 to 250 mg (3 to 5 tablets) per day, recommended divided into 3 doses; if it is necessary to take the drug at a dose of 250 mg, it is recommended to take 2 tablets. 50 mg morning and afternoon and 1 tablet. In the evening; in combination with levodopa drugs - 150 mg (3 tablets) per day, recommended divided into 3 doses.
When selecting a dose, if it is increased, it is recommended to titrate the dose, gradually increasing it by 1 table. (50 mg) every 2 weeks.
Indications
auxiliary symptomatic therapy for chronic impairment of cognitive functions and neurosensory deficits during the aging process (disorders of attention, memory, etc.);
Parkinson's disease: monotherapy (for forms predominantly involving tremor) and as part of combination therapy with levodopa both in the initial and later stages of the disease, especially in forms including tremor;
as an auxiliary symptomatic therapy for intermittent claudication resulting from obliterating diseases of the arteries of the lower extremities (stage 2 according to the Leriche and Fontaine classification);
treatment of symptoms of ophthalmological diseases of ischemic origin (decreased visual acuity, narrowing of the visual field, decreased color contrast, etc.).
Contraindications
increased individual sensitivity to piribedil and/or excipients included in the drug;
collapse;
acute myocardial infarction;
concomitant use with antipsychotics (except clozapine);
children under 18 years of age (due to lack of data).
With caution: due to the fact that the drug contains sucrose, patients with intolerance to fructose, glucose or galactose, as well as patients with sucrose isomaltase deficiency (a rare metabolic disorder), are not recommended to take the drug.