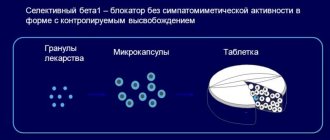
Betaloc ZOK, 100 mg, delayed-release, film-coated tablets, 30 pcs.
Metoprolol is a CYP2D6 substrate, and therefore drugs that inhibit CYP2D6 (quinidine, terbinafine, paroxetine, fluoxetine, sertraline, celecoxib, propafenone and diphenhydramine) may affect the plasma concentrations of metoprolol.
The combined use of Betalok® ZOK with the following drugs should be avoided.
Derivatives of barbituric acid.
Barbiturates (study conducted with pentobarbital) increase the metabolism of metoprolol due to enzyme induction.
Propaphenone.
When propafenone was prescribed to four patients treated with metoprolol, an increase in plasma concentrations of metoprolol was observed by 2-5 times, while two patients experienced side effects characteristic of metoprolol. This interaction was confirmed in a study of data from 8 volunteers. The interaction is likely due to the inhibition by propafenone, like quinidine, of the metabolism of metoprolol through the cytochrome P450 2D6 system. Taking into account the fact that propafenone has the properties of a beta-blocker, the joint administration of metoprolol and propafenone does not seem appropriate.
Verapamil.
The combination of β-blockers (atenolol, propranolol and pindolol) and verapamil can cause bradycardia and lead to a decrease in blood pressure. Verapamil and β-blockers have complementary inhibitory effects on AV conduction and sinus node function.
The combination of Betaloc® ZOK with the following drugs may require dose adjustment.
Amiodarone.
The combined use of amiodarone and metoprolol can lead to severe sinus bradycardia. Taking into account the extremely long T1/2 of amiodarone (50 days), the possible interaction should be considered long after discontinuation of amiodarone.
Class I antiarrhythmic drugs.
Class I antiarrhythmics and β-blockers may result in additive negative inotropic effects, which can lead to serious hemodynamic side effects in patients with impaired left ventricular function. This combination should also be avoided in patients with sick sinus syndrome and impaired AV conduction. The interaction is described using disopyramide as an example.
NSAIDs.
NSAIDs weaken the antihypertensive effect of β-blockers. This interaction has been documented for indomethacin. It is likely that the described interaction will not be observed with sulindac. Negative interactions have been noted in studies with diclofenac.
Diphenhydramine.
Diphenhydramine reduces the clearance of metoprolol to α-hydroxymetoprolol by 2.5 times. At the same time, an increase in the effect of metoprolol is observed.
Diltiazem.
Diltiazem and β-blockers mutually enhance the inhibitory effect on AV conduction and sinus node function. When metoprolol was combined with diltiazem, cases of severe bradycardia were observed.
Epinephrine (adrenaline).
Ten cases of severe hypertension and bradycardia have been reported in patients taking non-selective beta-blockers (including pindolol and propranolol) and receiving epinephrine (adrenaline). The interaction was also observed in the group of healthy volunteers. It is assumed that similar reactions can be observed when epinephrine is used together with local anesthetics if it accidentally enters the vascular bed. It is assumed that this risk is much lower with the use of cardioselective beta-blockers.
Phenylpropanolamine.
Phenylpropanolamine (norephedrine) in a single dose of 50 mg can cause an increase in blood pressure to pathological values in healthy volunteers. Propranolol mainly prevents the increase in blood pressure caused by phenylpropanolamine. However, β-blockers may cause paradoxical hypertension reactions in patients receiving high doses of phenylpropanolamine. Several cases of hypertensive crisis have been reported while taking phenylpropanolamine.
Quinidine.
Quinidine inhibits the metabolism of metoprolol in a special group of patients with rapid hydroxylation (in Sweden, approximately 90% of the population), causing mainly a significant increase in plasma concentrations of metoprolol and increased β-blockade. It is believed that a similar interaction is typical for other β-blockers, the metabolism of which involves cytochrome P450 2D6.
Clonidine.
Hypertensive reactions during abrupt withdrawal of clonidine may be exacerbated by concomitant use of beta-blockers. When used together, if clonidine is discontinued, discontinuation of beta-blockers should begin several days before discontinuation of clonidine.
Rifampicin.
Rifampicin may increase the metabolism of metoprolol, reducing plasma concentrations of metoprolol.
Patients concomitantly taking metoprolol and other beta-blockers (eye drops) or MAO inhibitors should be closely monitored. When taking β-blockers, inhalational anesthetics enhance the cardiodepressive effect. While taking β-blockers, patients receiving oral hypoglycemic agents may require dose adjustment of the latter.
Plasma concentrations of metoprolol may increase when taking cimetidine or hydralazine.
Cardiac glycosides, when used in combination with beta-blockers, can increase AV conduction time and cause bradycardia.
Indications for use of the drug Betaloc zok
- arterial hypertension (AH) (to reduce blood pressure and the risk of developing coronary and other cardiovascular complications, as well as cardiovascular and coronary death, including sudden death);
- angina pectoris;
- compensated chronic heart failure with impaired left ventricular systolic function (as an addition to the basic treatment of heart failure);
- in order to reduce mortality and the incidence of recurrent infarction after the acute phase of myocardial infarction;
- heart rhythm disturbances, including supraventricular tachycardia, as well as to reduce the frequency of ventricular contractions during atrial fibrillation and ventricular extrasystoles;
- functional disorders of cardiac activity;
- migraine prevention.
Overdose of the drug Betaloc zok
Symptoms: severe arterial hypotension, sinus bradycardia, AV block, heart failure, cardiogenic shock, cardiac arrest, bronchospasm, disturbances of consciousness up to coma, nausea, vomiting, cyanosis of the extremities. Concomitant use of alcohol, antihypertensive drugs, quinidine or barbiturates may worsen the patient's condition. The first symptoms develop 20 minutes to 2 hours after an overdose. Treatment: gastric lavage, taking activated carbon. In cases of severe arterial hypotension, bradycardia, or the threat of developing heart failure, administration of a β1-adrenergic receptor agonist (for example, prenalterol) intravenously at intervals of 2–5 minutes or as an infusion is indicated until a therapeutic effect is achieved. In the absence of a selective β1-adrenergic receptor agonist, it can be replaced by intravenous dopamine or atropine sulfate to block the vagus nerve. If a therapeutic effect cannot be achieved, other sympathomimetics (dobutamine or norepinephrine) can be used. Administration of glucagon at a dose of 1–10 mg is indicated. It may be necessary to use a pacemaker. To relieve bronchospasm, a β2-adrenergic receptor agonist is administered intravenously. It should be borne in mind that the doses of antidotes that are necessary to eliminate the symptoms of an overdose of a β-adrenergic receptor blocker are much higher than therapeutic doses, since β-adrenergic receptors are bound by their blockers.
Contraindications for Betaloc zok
AV blockade II–III degree; heart failure in the decompensation phase (pulmonary edema, hypoperfusion syndrome or arterial hypotension), simultaneous (long-term or periodic) therapy with inotropic agents aimed at stimulating β-adrenergic receptors; clinically significant sinus bradycardia, sick sinus syndrome, cardiogenic shock, severe peripheral arterial circulation disorders. Metoprolol should not be prescribed to patients with suspected acute myocardial infarction with a heart rate less than 45 per minute, a P-Q on the ECG of more than 0.24 s, or a systolic blood pressure level of less than 100 mmHg. Art. Hypersensitivity to any component of the drug or to other beta-adrenergic receptor blockers.
Use of the drug Betaloc zok
Betaloc ZOK is intended for daily use once a day, preferably in the morning. Tablets (or tablets split in half) should not be chewed or crushed. Food intake does not affect the bioavailability of the drug. During dose selection, heart rate should be monitored to prevent bradycardia. Hypertension (arterial hypertension) The recommended dose of Betaloc ZOK for patients with mild or moderate hypertension (arterial hypertension) is 50 mg 1 time per day. If the therapeutic effect is not achieved, the dose should be increased to 100–200 mg once a day or combined with other antihypertensive drugs. Angina pectoris The recommended dose is 100–200 mg Betaloc ZOK 1 time per day. If necessary, Betaloc ZOK can be combined with other drugs for the treatment of angina. Stable chronic heart failure with impaired left ventricular systolic function (as an addition to basic therapy) Patients must be in the stage of compensated chronic heart failure for at least 6 weeks; basic therapy should not change during the last 2 weeks. Treatment of heart failure with β-adrenergic blockers may lead to transient clinical deterioration. Further continuation of therapy or dose reduction is possible; in some cases, discontinuation of the drug may be necessary. Initiation of therapy with Betaloc ZOK in patients with severe heart failure (NYHA IV) should be carried out by an experienced physician experienced in treating patients with heart failure. Stable chronic heart failure, functional class II The recommended initial dose of Betaloc ZOK for the first 2 weeks is 25 mg (1 tablet of 25 mg or tablets of 50 mg) 1 time per day. After 2 weeks, the dose can be increased to 50 mg once a day and then can be doubled every 2 weeks. The optimal dose for long-term treatment is 200 mg Betaloc ZOK 1 time per day. Stable chronic heart failure, functional class III-IV The dose is selected individually. The recommended initial dose for the first 2 weeks is 12.5 mg Betaloc ZOK (1/2 tablet of 25 mg) 1 time per day. During the period of increasing the dose, the patient should be under the supervision of a physician, as in some cases the symptoms of heart failure may intensify. After 2 weeks of taking Betaloc ZOK at a dose of 12.5 mg, the dose can be increased to 25 mg (1 tablet of 25 mg or tablets of 50 mg) once a day. After 2 weeks, the dose can be increased to 50 mg once a day. For patients who tolerate higher doses well, the dose can be doubled every 2 weeks until a maximum dose of 200 mg Betaloc ZOK is reached once a day. In case of hypotension and/or bradycardia, it is necessary to reduce the dose of Betaloc ZOK or concomitant medications. Hypotension at the beginning of therapy does not necessarily indicate that such a dose of Betaloc ZOK will not be tolerated in the future. However, the dose should not be increased until the patient's condition has stabilized. Monitoring of kidney function is necessary. Cardiac arrhythmias The recommended dose is 100–200 mg Betaloc ZOK 1 time per day. Maintenance therapy after myocardial infarction It has been shown that as a result of long-term treatment with Betalok ZOK at a dose of 200 mg per day, the risk of death (including sudden death) is reduced, and the risk of recurrent myocardial infarction is reduced (including in patients with diabetes mellitus). Functional cardiac disorders accompanied by palpitations The recommended dose is 100 mg Betaloc ZOK 1 time per day. If necessary, the dose can be increased to 200 mg. Prevention of migraine The recommended dose is 100–200 mg Betaloc ZOK 1 time per day. Patients with impaired renal function No dose adjustment is required in patients with impaired renal function. Patients with impaired liver function Betaloc ZOK is usually prescribed to patients with liver cirrhosis at the same dose as patients with normal liver function. Only in case of severe liver failure is it possible to reduce the dose. Elderly patients No dose adjustment is required. Children Experience with the use of Betaloc ZOK in children is limited.
Side effects of the drug Betaloc zok
Well tolerated, side effects are usually mild and reversible. Side effects according to the frequency of occurrence are distributed as follows: very often - at least 10%, often - 1-9%, infrequently - 0.1%, rarely - 0.01-0.09%, very rarely - less than 0.01% . From the cardiovascular system Often: bradycardia, postural disturbances (extremely with dizziness), coldness of the extremities; uncommon: temporary worsening of symptoms of heart failure, 1st degree AV block, edema, pain in the heart area; rarely: sinoatrial conduction disturbance, arrhythmia; very rare: gangrene in patients with severe peripheral circulatory disorders. From the side of the central nervous system Very often: increased fatigue; often: dizziness, headache; uncommon: paresthesia, muscle cramps. From the gastrointestinal tract Often: nausea, abdominal pain, diarrhea, constipation; uncommon: vomiting; rarely: dry mouth. From the blood system : Very rare: thrombocytopenia. From the hepatobiliary system Rarely: changes in functional liver parameters; very rare: hepatitis. From the musculoskeletal system Very rare: arthralgia. Metabolic disorders : Uncommon: weight gain. Mental status: Uncommon: depression, decreased concentration, drowsiness or insomnia, nightmares; rarely: increased excitability, anxiety; very rarely: amnesia and other memory impairments, confusion, hallucinations. From the respiratory system Often: shortness of breath with physical effort; not often: bronchospasm; rarely: rhinitis. From the senses : Rarely: visual disturbances, dryness and/or irritation of the eyes, conjunctivitis; very rarely: taste disturbances, tinnitus. Skin disorders Uncommon: rash (urticaria, areas of skin dystrophy), increased sweating; rarely: hair loss; very rarely: photosensitivity, exacerbation of psoriasis. Other Impotence, sexual dysfunction.
Special instructions for the use of the drug Betaloc zok
Patients taking beta-blockers should not receive intravenous verapamil-type calcium antagonists. As a rule, when treating patients with asthma, β2-adrenergic receptor agonists (in tablets or aerosol) are prescribed as concomitant therapy. In cases where these patients begin to take Betaloc ZOK, an increase in the dose of β2-adrenergic receptor agonists may be necessary. The risk that Betaloc ZOK will affect β2-adrenergic receptors is lower than in the case of the use of conventional non-selective β1-adrenergic receptor blockers in tablets. Betaloc ZOK has a lesser effect on insulin release and carbohydrate metabolism than non-selective beta-adrenergic receptor blockers. In patients with chronic heart failure, compensation for the disease should be achieved before starting the use of Betaloc ZOK, and during its use they should be under medical supervision. In extremely rare cases, the condition of patients with moderate AV conduction disorders may worsen (possible development of complete AV block). If bradycardia develops during treatment, the dose of Betaloc ZOK should be reduced or the use of the drug should be gradually discontinued. Betaloc ZOK may increase the severity of peripheral arterial circulatory disorders by reducing blood pressure. Patients with pheochromocytoma should be prescribed an α-adrenergic receptor blocker simultaneously with Betaloc ZOK. When performing surgery, it is necessary to warn the anesthesiologist that the patient is taking Betaloc ZOK. However, it is not recommended to discontinue treatment with beta-adrenergic blockers in patients scheduled for surgery. Data on the effectiveness and safety of the drug in patients with severe stable heart failure (NYHA functional class IV) are limited. These patients must be treated by physicians with specialized skills and experience. Abrupt discontinuation of β-adrenergic blockers should be avoided, as this may worsen heart failure and also increase the risk of myocardial infarction and sudden cardiac death. If treatment must be stopped, this should be done as gradually as possible, over a period of at least 2 weeks under medical supervision. The dose is reduced by half at each stage. The last dose (12.5 mg) should be taken for at least 4 days until the drug is completely discontinued. If symptoms return, it is recommended to slow down the dose reduction. Anaphylactic shock in patients taking metoprolol is more severe. Pregnancy and lactation Betaloc ZOK can be prescribed during pregnancy only if the expected therapeutic effect for the mother outweighs the potential risk to the fetus. β-adrenergic receptor blockers can cause the development of bradycardia in the fetus and newborn, which should be taken into account when prescribing the drug in the third trimester of pregnancy, as well as during childbirth. It is unlikely that metoprolol prescribed to the mother in therapeutic doses will have a negative effect on the infant. Effect on the ability to drive vehicles and work with potentially dangerous mechanisms Since dizziness and weakness may develop when using the drug, caution should be exercised when driving vehicles and working with potentially dangerous mechanisms.