History of the discovery of interferon
Interferons (IFNs) belong to a special group of proteins that are produced by immune system cells in animals and humans. IFNs protect the body from pathogenic bacteria, parasites and even cancer cells. According to one version of scientists, thanks to interferons, humanity has not yet died from viral diseases.
The world learned about interferons not so long ago - only in the middle of the 20th century. And, despite the fact that many decades have passed since then, we can say that their discovery has become one of the most significant events in the medical world in the last and current century. In terms of scale, it is comparable only to the discovery of viruses themselves at the end of the 19th century.
Scientists were forced to think about the possible existence of some special protective barrier in the body by the phenomenon of interference, which consisted in the fact that after infection with certain viruses, cells become immune to viruses of another type. Scientists were most interested in the variant of interference in which the body, after the introduction of a non-pathogenic virus, became immune to infection by deadly pathogens. This method of protecting a person from diseases differs from the well-known vaccination, when by introducing a weakened or killed pathogenic agent they try to launch a specific system, i.e. acquired immunity.i
Interferons not only have antiviral activity (they were discovered by scientists for this reason), but also take a diverse role in the regulation of the immune response. Interferons as a system as a whole have a broader biological effect than limiting viral infection.ii
The mechanism of action of IFN is fundamentally different from antibodies. The fact is that IFNs are not specific to viral diseases and are able to fight different types of viruses. This means that medications containing interferons, which are intended for the prevention and treatment of acute respiratory viral infections, can fight not only a specific strain of influenza, for example, but all strains.
Solution
Interferon in ampoules for injection is produced in different dosages under different names: reaferon, wellferon, betaferon, etc. The products are available in syringe tubes, ready for use, which simplifies the use of the drug. Dosages in products – 500 – 10,000,000 IU.
You can also purchase interferon in powder form in glass ampoules. This is a lyophilisate that is produced using a special technology. It is dissolved in physiological solution for further use for intramuscular and subcutaneous injections. In this way you can fight any infectious lesions of the body.
An alternative way to use the powder is inhalation using a special drug - a nebulizer. It creates a cloud consisting of miniature particles. Such inhalations are considered effective in the treatment of respiratory diseases.
Classification of interferons
The name “interferon” comes from the English “to interfere”, which is quite consistent with the action of these proteins - they interfere, i.e. prevent infection.
Today, more than 20 types of human interferons are known, which can be divided into three types:
- The first type is antiviral interferons, which include interferon alpha (IFN-α), interferon beta (IFN-β), as well as some other interferons. The production of these IFNs begins when viruses, as well as some components of bacteria, enter the body. At the same time, other immune mechanisms are stimulated.
- The second type is the so-called immune IFN, interferon gamma (IFN-γ). It acts as a second level of protection, i.e. is included in the fight against the disease after the “first wave” and activation of type 1 IFN. The antiviral and antitumor properties of this IFN are less pronounced than those of the previous group of IFNs.
- The third type is represented by interferon lambda (IFN-λ), whose functions are still poorly understood. At the moment, its study is ongoing, but it has already been established that in its effect algorithm it resembles IFN alpha (IFN-α) and beta (IFN-β).
First production of interferon
For the first time, first-generation IFN preparations were obtained from leukocytes of donor blood. And immediately scientists began their clinical use and further study.
Science in the 20th century continuously developed, and over time, the basis for the biotechnological production of second-generation drugs containing recombinant IFNs was developed, i.e. obtained through genetic engineering.
Based on the method of production, IFN can be divided into 4 types:
- leukocyte IFN, which is obtained from donor blood;
- lymphoblastic IFN, obtained from the culture of lymphoblastic cells;
- recombinant IFN, which is obtained using bacterial or fungal cultures;
- pegylated types of IFN, which are obtained by combining recombinant IFN with a substance such as polyethylene glycol. In this way, pharmacists are trying to make the effect of the drug last longer.
The first production of IFN in the USSR was launched in 1988. At that time, these drugs were intended for the treatment of viral and oncological diseases. Great hopes were placed on interferons, so the study of these proteins was formed into a separate section of biology and medicine called interferonology. The scientific community actively began to develop a promising topic, as a result of which options for methods for determining the interferon status of the body were proposed.
At the moment, IFNs are considered as sufficiently studied immune mediators that have a wide range of biological activity and can be used not only in the prevention and treatment of viral diseases, but also non-viral diseases. Pharmacists have conducted many studies, with the help of which it was possible to prove that interferons occupy a leading position during therapy for such widespread and poorly controlled diseases as ARVI, hepatitis B, C and herpes.
Interferon therapy: yesterday, today and tomorrow
To conduct the first studies of IFN, a group of volunteers was recruited who agreed to inject themselves with IFN obtained from the blood of monkeys. In those days when IFNs were little studied, making such a decision required a certain amount of courage. Therefore, we can say that people agreed to sacrifice themselves for the sake of science. Before the introduction of IFN, they were infected with the Coxsackie virus, which causes the common cold.
After a certain period of time, it was found that only one person from the group of volunteers became ill, despite the fact that the virus was present in all of them in the nasal washes. However, no side effects were found. This study marked the beginning of thousands of subsequent experiments, thanks to which scientists recognized the ability of IFN to protect humans from disease.
At the same time, in the 1960s, Soviet virologists also joined the study of the effect of IFN on the human body. Epidemiologist Soloviev V.D. proposed to produce IFN from placental blood taken from postpartum women. In 1969, an epidemic of Hong Kong influenza was recorded in the USSR, which was first tried to be combated with the help of interferon preparations obtained from human blood. It was found that the effectiveness of such drugs is almost 70%.
IFNs were also widely used in subsequent years - for example, in 1973, when Academician A.A. Smorodintsev began researching this type of protein. In scientific works of those years, the most effective methods of using IFN were analyzed. Thus, through the ongoing study of IFN and its effect on the human immune system, by the end of the 20th century, scientists were able to accumulate enormous experience in the use of human leukocyte IFN for the prevention and treatment of respiratory infections, including influenza, as well as some other viral diseases.
Intranasal interferon preparations have been widely used in many countries around the world, including the UK, USA, Japan and Bulgaria. In recent years, interferon has begun to be widely used in the treatment of children. For adults, this group of drugs has been used for more than half a century. With the beginning of the use of IFN, the effectiveness of protection against ARVI in preschool children, including newborns, has increased significantly. This indicated to scientists that it is necessary to study IFNs more and more deeply and strive to expand the scope of their influence, to try to fight a variety of diseases with the help of IFNs.
However, unfortunately, after the start of mass production of IFN, it quickly became clear that the composition of drugs containing interferon is inconsistent even within the production of one pharmaceutical company. The main disadvantages of drugs obtained from donor blood include the inability to purify the drug at a high level. It turned out to be difficult to remove viral particles from the drug. In addition, donated blood is a very expensive ingredient, available in large quantities only to a few pharmaceutical companies. Therefore, the company has now almost abandoned the production of leukocyte IFN. A small volume of IFN preparations obtained from donor blood is used strictly for life-saving indications, when the expected benefit significantly outweighs the possible risks.
But did scientists really abandon the production of drugs with interferons after learning about the peculiarities of leukocyte IFN? Of course not. In this case, biotechnology came to the rescue - the science of ways to create various substances using natural biological components. Thanks to it, today pharmacists produce recombinant IFNs, absolutely identical to natural ones in amino acid composition, which do not require donor blood. These drugs are produced in large quantities throughout the world and are relatively low in cost, which in turn makes prices in pharmacies quite affordable for most consumers.
IFN preparations today can be produced in the form of dried lyophysiate, which is subsequently used to obtain a solution, suppositories for rectal and vaginal use, a solution for injections and inhalations, drops, ointments, gels, aerosols, tablets and microenemas.
IFN drugs may contain different amounts of the active substance. On drug packaging, this amount is most often indicated in international units, or IU.i
The release form and dosage of the IFN drug is selected by the attending physician, taking into account the patient’s age, condition and medical history.
Are medications containing interferons suitable for children?
With acute respiratory viral infections and other viral diseases, children often experience a deficiency of interferons. For this reason, an additional source of IFN is needed to speed up recovery. Interferon, coming from outside, helps to mobilize the immune system of children and protects them from the development of complications.
Frequently ill children (FIC) require special attention, and this group includes not only preschoolers and schoolchildren who suffer from flu and colds more than 4-6 times a year, but also those who are ill for a long time - more than two weeks after each infection with ARVI. In such children, the production of natural IFN is reduced by no less than 15-20% compared to their peers. Medicines containing IFN not only fight viral diseases, but also enhance protective functions and help resist the disease.
For PBD, pediatricians recommend the use of long-term interferon therapy. Using this approach, it is possible to form a special IFN depot, due to which the cells in the body become resistant to the effects of infections.
Introduction
It is known that acute respiratory viral infections (ARVI), caused by pathogens belonging to at least 6 families (orthomyxo-, paramyxo-, picorna-, corona-, parvo-, adeno-) viruses, firmly occupy a leading place in the structure of infectious diseases, especially in pediatric infectious pathology.
In Russia, ARVI is registered annually at approximately 70–80 thousand diseases per 100 thousand children (3.3 times higher than in adults) with no downward trend. The frequency of occurrence of influenza cases is often observed less frequently than diseases of other etiologies in total, even during its seasonal epidemic rise. And only in patients of any age with a severe form of infection do diseases caused by influenza viruses dominate: currently, predominantly the causative agent of the 2009 pandemic is the influenza virus type A (H1N1) pdm 2009 [1–3]. In approximately 25–30% of cases, especially in newly created groups (a group of a preschool institution, the first grade of a school, a hospital ward, a conscript barracks), there is simultaneous participation of several pathogens in the infectious process due to the “mixing” effect.
It has also been established that acute respiratory viral infections, despite the obligatory damage to the mucous membranes of the nasopharynx and upper respiratory tract, are distinguished by significant polymorphism of clinical symptoms, and most of their pathogens, except influenza viruses, are characterized by a lack of sensitivity to modern popular antiviral drugs: neuraminidase inhibitors oseltamivir and zanamivir, used in currently in the treatment of influenza [4].
In addition, the use of these drugs is possible only in the treatment of patients older than 1 year (oseltamivir) and from 5 years of age (zanamivir), and the fusion inhibitor umifenovir - for all acute respiratory viral infections, but in children no earlier than 2 years of age [5].
Statistics
According to data from the Federal Service for Surveillance in the Sphere of Consumer Rights Protection and Human Welfare, it follows that the number of children under the age of 17 who fell ill with ARVI in 2016 in the Russian Federation was 2.64 times greater than the number of adults who fell ill, and in 2022 - 2 ,71, in 2018 - 2.51 times, and in terms of per 100 thousand population of the corresponding age, the number of acute respiratory viral infections in children under 17 years of age is 3.71 times greater than the number of all cases in 2016, in 2022 - by 3.73, in 2022 - by 3.58 times, with the largest number of cases being in the age group under 14 years (Table 1).
It should be noted that the frequency of registration of influenza both in the entire population of the country and in both age groups of children during the described period of time was significantly less common than acute respiratory viral infections of other etiologies (per 100 thousand of the total population: in 2016 - 357.4 times, in 2022 – 622.3 times, in 2022 – 795.2 times) [6, 7]. The number of registered cases of influenza in children (in terms of 100 thousand) in 2016 was 2 (under the age of 17 years) and 3 times more (under the age of 14 years), in 2022 - 3 times more in both groups, in 2018 - approximately 2.5 times more than in the entire population.
The frequency of registration of community-acquired pneumonia in children (in terms of 100 thousand) was also higher (in 2016 - 1.7 and 1.9 times; in 2022 - 1.7 and 2.0; in 2022 g. - 1.9 and 2.0 times) than that of the entire population as a whole.
Somewhat different data were obtained by analyzing the frequency of registration of verified influenza among 10,399 patients hospitalized for this disease in 3 infectious diseases hospitals (2 for children and 1 for adults) in St. Petersburg (base of the Federal State Budgetary Institution Research Institute of Influenza) during seasonal (from November of this year to May next) epidemic rises of influenza and ARVI during 2015–2019. (Table 2). The etiology of diseases was determined using polymerase chain reaction (PCR) in materials from the mucous membrane of the nasal passages or nasopharynx of patients [8].
In general, the proportion of patients with verified influenza (mono+mixed) was only 33.1% of the entire observed population, incl. 39.6% in the 2015–2016 season, 26.3% in the 2016–2017 season, 28.1% in the 2017–2018 season. and 38.9% in the 2018–2019 season. However, the proportion of influenza in hospitalized children was slightly lower (29.3% in the 2015–2016 season, 22.5% in the 2016–2017 season, 21.8% in the 2017–2018 season . and 38.6% - in the 2018–2019 season), which statistically significantly, except for the 2018–2019 season, differed from the data obtained from the adult contingent (57.1% in the 2015–2016 season, 35 .9% in the 2016–2017 season, 40.4% in the 2017–2018 season and 39.7% in the 2018–2019 season). At the same time, the total number of hospitalized patients with ILI, i.e. ARVI of various etiologies (PCR+ and PCR- in total) in all observed epidemic seasons prevailed over the number of patients with verified influenza, especially among children. It also turned out that, in general, the number of children admitted for inpatient treatment always statistically significantly dominated the number of adults. At the same time, in some epidemic seasons, in particular in the 2015–2016 season, the number of adult patients with verified influenza exceeded the number of children.
It was shown that the main contingent of hospitalized children in the analyzed period of time belonged to the age group 0–2 years (978/51.4% of 1905 people in the 2015–2016 season, 1251/62.7% of 1997 people in the 2016–2016 season). 2017, 1089/63.8% of 1708 people in the 2017–2018 season and 749/60.2% of 1245 in the 2018–2019 season (Table 3, Fig. 1).
The number of hospitalized schoolchildren aged 7–17 years was the smallest (360/18.9% of 1905 people in 2016, 295/14.8% of 1997 people in the 2017–2018 season and 206/12.1% of 1708 people in 2018). Moreover, in all age groups of children, the number of cases of ILI (PCR+ and PCR- in total) prevailed over the number of cases with verified influenza.
Among the adult population, young patients aged 18–49 years dominated (944/84.7% of 1115 people in 2016, 727/90.2% of 806 people in 2022 and 607/71.7% of 847 people in 2022) (Table 4). Hospitalized patients aged 61 years and older accounted for only 7.4% in 2016, 4.0% in 2022, but 15.4% in 2018.
Etiology of ILI
The etiological structure of influenza in the observed patients during the 2015–2016 epidemic season. 4 years after the first post-pandemic epidemic season, the dominance of the influenza virus type A(H3N2) was again dominated by the influenza virus type A(H1N1) pdm 09, which coincided with the data obtained in the seasons of 2009–2010 (pandemic) and 2010–2011 ., which was also observed during the epidemic rise of influenza in the 2018–2019 season. (Fig. 2).
Among the causative agents of ILI (ARVI), the RS virus dominated in the child population (in every 3rd child), mainly at the age of the first two years, then rhino- and adenoviruses. Parainfluenza virus was more often detected in individuals with acute stenosing laryngotracheitis (ASLT). In adults, adeno- and rhinoviruses were detected more often than others (Fig. 3).
It has been proven that the severity and speed of development of clinical manifestations of infectious diseases, incl. and ARVI, are caused both by the properties of the pathogen and the massiveness of the invasion, and by the activity of immune defense factors aimed at limiting the reproduction of the pathogen, its elimination and restoration of structural and functional disorders in the patient’s body.
Antiviral protection
The leading role in antiviral protection in the first stages of the disease belongs to the interferon (IFN) system – natural cytokines that have universal antiviral properties: the ability to suppress the reproduction of many RNA and DNA viruses [9].
The presence of this system in a living organism was proven after the discovery in 1957 by A. Isaacs and J. Lindenmann of the IFN protein as a factor determining the phenomenon of viral interference (the ability to delay the development of viruses in cells) - nonspecific resistance induced by the viruses themselves and extending not only to inducer virus, but also to other agents [10]. The authors described IFN as “a protein, much smaller than immunoglobulins, that is produced by the cells of various animal species after infection with live or inactivated viruses; capable of inhibiting the growth of a variety of viruses in the cells of the same animal species in doses that are not toxic to cells.” Already in 1959, a Scientific Committee on IFN was formed to coordinate the scientific research program, headed by A. Isaacs.
In various countries, work has begun actively to study the properties of this protein and the possibility of using it to protect against viral infection. The first successful experiment with monkey IFN on volunteers was carried out in 1960 by DA Tyrrell [11].
In the USSR, and then in Russia, research successfully proceeded in two directions:
- The study of the possibility of IFN induction by live vaccines, which began at the Influenza Research Institute of the Russian Academy of Medical Sciences (interferon laboratory) under the leadership of A.A. Smorodintsev with the participation of the author of this communication [12, 13].
- Development of the production basis and creation of a leukocyte IFN preparation on human leukocytes using the original technology proposed by V.D. Solovyov (Gamaleya Institute of Epidemiology and Microbiology) [14].
The results of the developments of both research teams were successfully used during the epidemic of influenza type A (H3N2) Hong Kong in 1969, and the technology for the production of the leukocyte IFN drug was subsequently used both in our country and in other countries (Bulgaria, Japan, Great Britain , USA) [15–17].
At the same time, despite the successful use of leukocyte IFN, its disadvantages, like other drugs obtained from human blood, in addition to the heterogeneity of the composition, include a low degree of purification, especially from viral particles - inducers of IFN synthesis. Currently, leukocyte IFN drugs are used strictly for life-saving indications, when the expected benefit outweighs the possible risk.
The production of human IFN-α preparations in bacterial cells by genetic engineering methods made it possible to overcome all the above-mentioned problems and obtain pure, homogeneous recombinant IFN protein, identical in amino acid composition to its natural one, in large quantities and at a relatively low cost [11].
In the 1970s Types I and II of IFN, produced by different cells under the influence of different stimuli, have been described. In the mid-1980s. The concepts of “interferon status” and “interferon deficiency states” were formed, and numerous effects of IFN were discovered, associated not only with antiviral, but also with antiproliferative activity [18, 19].
The concept of the presence of the IFN system as an integral part of the immune system was finally formulated in the mid-1990s, and its direct and inverse connections with the immune and neuroendocrine systems of the body were discovered. If the immune system has specialized organs and cells, and its main function is to control the protein constancy of the body and a specific response to foreign antigenic information, then the IFN system, on the contrary, is disseminated throughout almost all cells of the body and has only relative species specificity, but it plays a leading role in the supervision of the genetic constancy of cellular structures, aimed at recognizing and eliminating foreign genetic information. IFN genes are inducible, i.e. To turn on their activity and, accordingly, the synthesis and secretion of IFN, activation of IFN-producing cells is required. Antigens acting on membrane TL receptors of macrophages, fibroblasts, T-lymphocytes and other cells act as inducers of the production of different types of IFN. The main inducers of type I IFN are double-stranded and single-stranded viral RNA, which exerts its action through TLR3 and the TLR7/TLR8 combination, as well as bacterial DNA. Some bacterial products, in particular lipopolysaccharide, can be inducers of type I IFN [20].
Depending on the immunogenic properties and differences in the gene sequences encoding different populations of IFN molecules, as well as on the physicochemical characteristics and mechanism of their action, 3 types of IFN are distinguished, of which the most significant is type I (IFN-α and IFN-β). It is these types of IFNs that are predominantly included in the drugs used in clinical practice.
Type I IFN (acid-stable): α – IFN-α (13 isoforms – 1, 2, 4, 5, 6, 7, 8, 10, 13, 14, 16, 17, 21), β – IFN-β (β1 and 3 β2), ω – IFN-ω, ε – IFN-ε and κ – IFN-κ. The genes for type I IFN, like type II BAY, are located on chromosome 9 [21]. There are also IFN-δ (limitin) – IFN-δ and zeta isoforms not found in humans. The main source of type I IFN is plasmacytoid precursors of dendritic cells, as well as monocytes and macrophages. In addition, IFN-α is secreted by epithelial cells and fibroblasts, and during viral infection - by all nucleated cells. The highest level of IFN-α synthesis was found in brain tissue, the lowest – in skeletal and cardiac muscles [17–19].
The main producers of IFN-β are fibroblasts and epithelial cells, as well as monocytes and macrophages.
The main biological properties of type I IFN (IFN-α and IFN-β) include direct antiviral activity (blocking the transcription and translation of viruses) and suppression of cell proliferation, which is necessary to prevent the spread of the virus, as well as stimulation of the functions of natural killer (NK) cells and cytotoxic lymphocytes that cause lysis of virus-infected target cells. The mechanisms of both innate (NK cells) and adaptive (T lymphocytes) immunity are activated. Therefore, all type I IFNs have, in addition to antiviral immunoregulatory activity. They also protect the genetic information of host cells from genome changes that can be caused by viruses, and also limit the proliferation of damaged and aging cells, inhibiting the functions of stem cells. There are practically no cells that are unable to respond to the action of type I IFN. In addition, it has been proven that they regulate the processes of lipid peroxidation, help restore disturbed homeostasis, and also accelerate the production of antibodies [22, 23]. As a result of the above, surrounding uninfected cells are protected by newly formed IFN, which prevents the spread of infection.
Type II IFN – IFN-γ is unstable at pH=2.0. IFN-γ is synthesized and secreted primarily by activated T lymphocytes and natural killer (NK) cells. The possibility of its formation by myeloid cells (monocytes/macrophages), dendritic cells and B lymphocytes has also been shown. IFN-γ activates macrophages and supports the proliferation of cytotoxic T lymphocytes. It is at this stage that mediators such as superoxide (O2), nitric oxide (NO), the cytokines interleukin-12 (IL-12) and tumor necrosis factor α (TNF-α) occur as part of a cascade of other pro-inflammatory and anti-inflammatory cytokines.
Type III IFN – λ (1. IFN-λ1/IL-29, 2. IFN-λ2/IL-28A, 3. IFN-λ3/IL-28B). Their genetic structure, located on chromosome 19, was first deciphered in 2002.
They are a distinct family of cytokines that are structurally and genetically distinct from type I IFN and use a separate receptor system, but their biological activity is similar to type I IFN [24].
It has been established that the kinetics of production of different types of IFN is determined by the time parameters of activation of the corresponding immunocompetent cells (ICC) [25–27].
It has been shown that the IFN system is the leading link of the immune system in the body's antiviral defense. IFN begins to be synthesized from the first hours of infection, before any other defense mechanisms are activated, and its level both at the entrance gates of infection and in the general circulation depends on the individual genetic characteristics of the host organism and on the properties of the pathogen that caused the pathological process. Geometric mean titers of IFN in the blood during ARVI of non-influenza etiology, and especially during respiratory syncytial (RS) viral infection, were 2–3 times lower than during influenza [27–30].
In patients with influenza complicated by pneumonia, as well as in its protracted course, by the period of convalescence, a decrease in the value of all IFN-α indicators was recorded, which is associated with the development of asthenic syndrome and is evidence of the advisability of using drugs with immunostimulating activity during this period [31].
The greatest significance of the indicators of IFN-α and -γ-producing (IP) ability of the ICC has been proven, and its dependence in patients with ARVI on the premorbid background of the patients has been revealed. Lower rates were recorded for all background pathologies, incl. and in frequently ill children (FIC) with cutaneous or respiratory allergy history [32]. So, according to E.A. Erman (2009), a decrease in the induced production of IFN-α was detected in 34.4% of cases among BBD, and in the production of IFN-γ - among 73.1% of those examined. At the same time, the content of circulating IFN in the blood serum of 100% of the examined BBDs was characterized by normal values [33].
Forms of recombinant IFN preparations
Various dosage forms of recombinant IFN preparations with a wide range of applications have been developed and introduced into healthcare practice: injection forms, eye drops and films, rectal and vaginal suppositories, microenemas, ointments and gels for dermatological use, aerosols, solutions and tablets for oral use, the most the most significant of which were recombinant IFN-α-2 preparations.
If chemotherapy drugs are mainly only means of etiotropic therapy, then IFNs have a combined effect (etiotropic and immunomodulatory). They do not have the disadvantages of chemotherapy drugs (narrow spectrum of action, development of resistance), and the spectrum of antiviral activity of these drugs is very wide: influenza and all other acute respiratory viral infections, herpetic infections, etc. More than 90.0% of diseases caused by viruses can be treated by including intranasally administered IFN in therapy [34].
But the most optimal for the treatment and prevention of ARVI of various etiologies in patients of any age (including newborns, pregnant and lactating women) is the use of recombinant IFN-α-2b. Their effectiveness increases if the drugs are used from the moment the first symptoms of the disease appear.
Grippferon
The drug of choice is the domestic original drug of recombinant IFN-α-2b Grippferon (FIRN M LLC, Moscow) with the optimal intranasal method of administration (patent No. 2140285 in the State Register of Inventions of the Russian Federation) [35]. The active ingredient of the drug is human recombinant IFN-α-2b, which is obtained from the biomass of Escherichia coli bacteria, into the genetic apparatus of which the human IFN-α-2b gene is integrated (FSP P N000089/01-050 111 - nasal drops, FSP LP0015-03- 311017 – nasal spray). 1 ml of the drug contains at least 10 thousand IU of IFN, as well as the antioxidant Trilon-B and the biologically compatible polymer povidone. The drug acts at the entrance gate of respiratory pathogens - the epithelial cells of the nasal mucosa. Polymer compounds help fix the drug on the mucous membrane and restore the patency of the nasal passages. In vivo and in vitro experiments have proven that povidone itself has the ability to stimulate the production of IFN.
The virus inhibitory effect of the drug Grippferon with a stable positive result was proven by employees of the Federal State Budgetary Institution Research Institute of Influenza named after. A.A. Smorodintsev Ministry of Health of the Russian Federation, St. Petersburg Research Institute of Epidemiology and Microbiology named after. Pasteur and the Virological Center of the Research Institute of Microbiology of the Ministry of Defense of the Russian Federation on the orthomyxovirus model, incl. avian influenza type A (H5N1 and H5N2), influenza type A (H1N1) pdm 2009, paramyxo- (parainfluenza and MS), adeno- and coronavirus infections, as well as rubella virus in various cell cultures and in experiments on laboratory animals, and this the effect in some cases was more pronounced than that of the comparison drug [36–38]. A stable suppression of viral replication without toxic damage to cells was achieved. The drug delayed the reproduction of these viruses when used both as a prophylactic and therapeutic regimen.
In 2013, as a result of an in vitro study on Vero cell culture and in vivo experiments on mice, employees of the Federal Budgetary Institution State Research Center for Virology and Virology "VECTOR" showed that recombinant IFN-α-2b has antiviral activity against SARS coronaviruses (hCoV strain Urbani) and MERS (hCoV-EMC/2012). The drug reduced the viral load in the lungs of mice by 10 times on the third day after infection with the wild SARS virus (hCoV strain Urbani) both in emergency prophylactic and therapeutic regimens [39].
Parallel clinical studies to study the therapeutic effectiveness of recombinant IFN-α-2b in children hospitalized for influenza and ARVI were conducted in the autumn-winter period of 2002 by employees of the Federal State Budgetary Institution Research Institute of Influenza of the Ministry of Health of the Russian Federation, St. Petersburg (in 155 children) and MONIKI , Moscow (240 people). In total, 230 patients received the drug; the comparison group included 165 children. Observations have shown that intranasal administration of the tested drug in the early stages of the disease to children, incl. and infancy (70% of the population) contributes to a more rapid elimination of all symptoms of the disease (Fig. 4) and a statistically significant reduction in the period of release of viral antigens found in patients in the nasal passages for 7 or more days after hospitalization and initiation of treatment [26, 34 40–44]. The positive effect of taking the drug on the dynamics of the temperature reaction and the severity of intoxication, incl. and in children of the first year of life. If before the start of treatment its structure in children in the compared groups was almost the same with a predominance of cases of diseases with an increase in temperature ≥38.0°C, then the very next day after using the drug the number of cases of ARVI with elevated body temperature and significant intoxication decreased statistically significantly ( 63.3 and 89.2%, respectively), especially due to a decrease in the frequency of this febrile reaction (Fig. 5).
The use of recombinant IFN-α-2b did not cause any subjective complaints in children or their parents and was not accompanied by the development of side clinical effects. The administration of the drug was accompanied by the restoration of the secretory immunoglobulin A (sIgA) content in nasal secretions, which was reduced at the onset of the disease in most children, in contrast to children in the comparison groups, among whom in every second examined the value of this indicator continued to decrease. Positive results were recorded in all observed patients, regardless of the level of their serum IFN-α content and IFN-producing activity of ICC in vitro, incl. and in CHBD, HIV-infected people and in children with perinatal contact with HIV infection (34, 42, 43).
During 2004–2005 in two centers: Saratov Medical University and MHPU "City Hospital No. 33" of Nizhny Novgorod - the base of the State Educational Institution of Higher Professional Education Nizhny. State Medical Academy of Roszdrav, a multicenter randomized study was conducted to study the possibility of using recombinant IFN-α-2b in the treatment of influenza and ARVI of other etiologies in pregnant women. Intranasal use of the drug according to the treatment regimen in the early stages of the disease contributed to a faster recovery of patients due to a statistically significant reduction in the duration of almost all symptoms of damage to the respiratory tract, incl. and febrile reaction (Fig. 6) [43, 45].
The therapeutic effect of recombinant IFN-α-2b in patients with influenza (adults, including pregnant women, and children) began already on the 2nd day of treatment, manifested by the disappearance of catarrhal symptoms in the nasopharynx, a decrease in body temperature and an improvement in the general condition of the patients . In general, the duration of the disease in patients with the drug was reduced by 2 days.
The administration of the drug contributed to a reduction in the period of isolation of viral antigens, and in children of the main groups, new pathogens were practically not detected during repeated IF examinations, which contributed to a rarer (2.5–3.0 times) development of repeated (2.5–3.0 times) than in children of the comparison groups. nosocomial) ARVI (from 23.0% of patients among children who did not receive the drug to 8.9% in the main group), occurring both in the hospital and shortly after the child was discharged home (Table 5).
At the same time, clinical studies were conducted in organized groups of children and adults to study the preventive activity of recombinant IFN-α-2b (Grippferon).
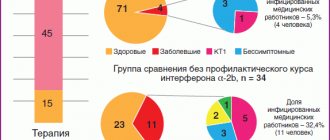
Emergency prevention of ARVI in organized preschool institutions of St. Petersburg with the help of the drug was carried out by employees of the Influenza Research Institute during local outbreaks, the etiology of which was determined using real-time PCR. Three outbreaks were caused by an adenovirus, in two cases by a combination of an adenovirus with influenza A(H3N2) and parainfluenza viruses, and only one outbreak was caused by a combination of influenza A(H3N2) and parainfluenza viruses [33, 34, 38]. An analysis of the incidence of 396 children aged 1–6 years during the entire observation period (1 year) showed that the differences in the frequency of ARVI registrations among children who received the drug (n=218) and in the compared groups (n=178) were statistically significant (Table 6).
Among those who received the drug, 59.2% of children never got sick, in the comparison group – 16.8% (p <0.001). The efficiency index (IE) of recombinant IFN-α-2b was generally 2.3, and the efficiency factor (EI) was 52.8. ARVI in children of the main group occurred mainly in the form of rhinitis and nasopharyngitis against the background of normal or subfebrile body temperature without intoxication and complications, which were observed in 6.6% of cases in children of the comparison groups. It should also be noted that the duration of identified ARVI symptoms was statistically significantly shorter in children receiving the drug compared to the comparison group.
A successful study was the preventive effectiveness of the drug in pregnant women [45, 46] and premature babies born with very low and extremely low body weight, who suffered a critical condition and were transferred from the intensive care unit (Ivanovo Federal State Budgetary Institution Research Institute of Maternity and Childhood) [47].
In children receiving the drug (nasal drops), acute respiratory viral infections developed less frequently, were milder with fewer complications (in 3.5% of cases versus 32.2% in the comparison group; p <0.001) and were statistically significantly shorter in duration (10, 0±1.3 versus 13.2±5.6 days in the comparison group; p<0.001). The risk of influenza in pregnant women receiving the drug decreased by 2.6–3.0 times, and among children receiving the drug, 47.4% did not get sick versus 13.6% of premature infants from the comparison group.
The results obtained indicated the possibility of using this drug for therapeutic and prophylactic purposes by children, incl. and the first year of life, with influenza and other acute respiratory viral infections, which was confirmed by numerous observations in children's and adult groups in Russia (Moscow, Moscow region, St. Petersburg, Yaroslavl, Ulyanovsk, Nizhny Novgorod, Saratov) during epidemic rises of influenza for 2001–2019 years, in which a statistically significant (2.4–3.5 times) decrease in morbidity was recorded among individuals receiving the drug. Similar results were recorded in “risk” groups, in particular among medical workers and pharmacists, railway employees, in groups of disabled and elderly people, as well as children of different ages, incl. and frequently ill people who lived in institutions of the Committee for Social Protection of the Population [34, 42–44]. The drug was most successful when taken at least a few days before the onset of the disease outbreak.
Conclusion
Thus, laboratory studies (on cell cultures in vitro and on animals in vivo), as well as numerous clinical studies of the domestic drug recombinant IFN-α-2b Grippferon (nasal drops and dosed nasal spray) allow us to consider it as a safe, effective preventive and therapeutic remedies for ARVI of any etiology and influenza in all age groups of patients, including newborns, incl. and premature and elderly patients. The drug is successfully used in pregnant and lactating women, as well as in children with concomitant background diseases and who are often ill.
Are all “pherons” the same?
As already mentioned, some drug manufacturers produce drugs whose names contain the ending “...feron,” which can mislead the consumer and convince him that the drug contains interferons. In fact, these drugs may be homeopathic and do not contain IFN in any form. Homeopathy refers to alternative forms of medicine that involve the use of highly diluted drugs that are believed to cause disease-like symptoms in healthy people. Proponents of homeopathy contrast the concept of treatment according to the principle “like cures like” with the principles of rational pharmacotherapy.
Currently, homeopathic medicines may not undergo a full cycle of checks before being entered into the RLS (register of medicines), since a simplified registration form applies to them.
In 2022, scientists of the Russian Academy of Sciences (RAS) recognized homeopathy as a pseudoscience and came to the conclusion that its use in medicine “contradicts the main goals of domestic healthcare and should be met with organized government opposition.”
If you do not want to make a mistake, then before purchasing the medicine at the pharmacy, you should read the instructions and make sure that IFN is present in the composition. Trust reliable brands and pharmaceutical companies that have been known on the market for many years and have already won the trust of consumers.
Drugs that act on the immune system: what is the difference?
Immune drugs refer to special drugs that are of biological, plant or synthetic origin and can affect the immune system. The strength of their influence depends on the initial state of human immunity. In addition to modulators, immune drugs also include immunostimulants and immunosuppressants.
Immunostimulants strengthen the body's defenses against a particular infection. Those. the body is forced to resist, significantly strengthening cellular and humoral immunity. A distinctive feature of these drugs is the indiscriminate action of the immune system - a “shake-up” of the immune system. That is why this group of drugs must be used under the strict supervision of a doctor.
Immunosuppressants are designed to suppress the immune response. After all, sometimes immunity does not work for a person’s benefit, and then autoimmune diseases develop, in which the body’s own immune system destroys organs and tissues of the body. Immunosuppressants are also used during organ transplants to prevent rejection.
Immunomodulators are drugs that can have a targeted effect on a person’s immunity: they can increase low levels and decrease high levels, thus bringing the defenses back to normal. This is their main difference from immunostimulants and immunosuppressants. Medicines containing interferon are indicated for identified immunodeficiency of any etiology, for the treatment and prevention of various infectious and inflammatory diseases. Already today, many doctors advise the use of antiviral drugs with interferon to prevent and treat COVID-19 infection, as indicated by the national guidelines of the Ministry of Health of the Russian Federation.
The main criterion according to which a doctor prescribes immunomodulators is the clinical picture of the disease, which manifests itself as a chronic infectious-inflammatory process. Immunomodulators are also used to prevent a number of diseases.
Sources
- Malinovskaya V.V., Chebotareva T.A., Parfenov V.V. Clinical effectiveness of the drug Viferon in the treatment of influenza and ARVI in adults // Almanac of Clinical Medicine. 2014. No. 35. pp. 109–115
- Malinovskaya V.V., Korzhov I.V., Mosyagin I.G. Current aspects of antiviral therapy for ARVI and influenza in military groups // Marine Medicine. 2022. T. 6, No. 1. P. 15–00, https://dx.doi.org/10.22328/2413-5747-2019-5-4-15-23.
- McKenzie C. Ferguson and all. Interferons as Therapeutic Agents in Infectious Diseases // Pharmacy Faculty Research, Scholarship, and Creative Activity. -2011
Fan-ching Lin and Howard A. Young. Interferons: Success in anti-viral immunotherapy // Cytokine Growth Factor Rev. 2014 Aug; 25(4): 369–376. doi: 10.1016/j.cytogfr.2014.07.015
Interferon alpha 2 b as part of the antiviral drug VIFERON
The drug VIFERON is one of the antiviral drugs that have a wide spectrum of antiviral activity and help restore immunity. This drug contains recombinant interferon alpha-2b, which prevents the spread and reproduction of viruses in the body. It is identical to human interferon alpha-2b, which is synthesized naturally in human cells in response to foreign agents, but is produced using modern technology without the use of donor blood. Recombinant interferons belong to a group of antiviral drugs that are used for therapeutic and prophylactic purposes.
The drug in the form of suppositories is used in children and adults as part of complex therapy for the treatment of many diseases. When using the drug in the form of suppositories, the liver and stomach do not experience additional stress, which is very important for young children, people suffering from gastrointestinal diseases, including gastritis, as well as for older people.
It is worth noting that VIFERON does not belong to homeopathic remedies, but is a preparation of classical medicine. That is, to begin production, it had to go through all stages of clinical trials, as well as all kinds of quality checks (more than 250 indicators).
Interferon alpha 2b human recombinant as part of the drug VIFERON Gel
VIFERON Gel containing interferon alpha-2b is used for the prevention and treatment of acute respiratory viral infections, frequent and long-term acute respiratory viral infections, including those complicated by bacterial infection. In addition, the drug is used for the prevention and treatment of recurrent stenosing laryngotracheobronchitis - inflammation of the mucous membrane of the larynx and trachea, which leads to narrowing of the airway lumen and disruption of their functioning. When applied to the skin, mucous membrane and tonsils, the gel forms a thin protective layer, which prevents the penetration of viruses and also strengthens local immunity.
Also, the drug VIFERON Gel is used to treat acute and chronic herpetic infections of the skin and mucous membranes, including its urogenital form.
Interferon alpha 2 b as part of the drug VIFERON Ointment, instructions for use
VIFERON Ointment is an immunomodulatory and antiviral agent for external and local use. The ointment is produced in the form of a yellow or yellowish-white substance, viscous, homogeneous, with a specific odor of lanolin. The drug contains human recombinant interferon alpha-2b, tocopherol acetate, anhydrous lanolin, petroleum jelly, purified water and peach oil.
The drug VIFERON Ointment is used to treat ARVI, including influenza, as well as herpes of various localizations. For herpes (Herpes simplex types 1 and 2), the use of ointment leads to the complete disappearance of skin rashes.iii
Reference and information material
Shamsheva Daria Sergeevna
General practitioner, cardiologist, Ph.D.
Sources:
i
ii https://humbio.ru/
iii A.A. Khaldin, O.V. Chistik, D.V. Ignatiev “Herpes simplex: etiology, pathogenesis, diagnosis, treatment”, Practical Medicine No. 5, 2009
Loading...
Take other surveys
Interferon-based drugs for the prevention of ARVI
In most countries of the northern hemisphere, ARVI, that is, acute respiratory viral infections, is one of the most widespread infectious diseases. Despite the popularization of vaccination aimed at protecting against influenza, it cannot fully solve the problem of ARVI. The thing is that vaccinated people develop antibodies only to those strains of the influenza virus that are part of the vaccine. But viruses constantly mutate, so you may be offered a vaccine against a certain number of influenza strains, but you will get sick from completely different strains. Scientists cannot create vaccines as quickly as mutation occurs. It is also necessary to take into account the fact that vaccines are not used in the midst of an epidemic.
Preventive protection against ARVI must be comprehensive, including nonspecific prophylaxis, which is not aimed at specific strains, but acts against the majority of ARVI known to science. It is necessary to use antiviral drugs that have a significant impact on preventing the occurrence of diseases and on the favorable outcome of the developed disease.