Sodium bicarbonate
(lat. Natrii hydrocarbonas), other names:
sodium bicarbonate
,
tea garden
,
drinking
or
baking soda
,
sodium bicarbonate
- an inorganic compound, sodium acid salt of carbonic acid with the chemical formula NaHCO3.
In its usual form it is a finely crystalline white powder.
It is used in industry, the food industry, cooking, and medicine as a neutralizer of chemical burns of the skin and mucous membranes with concentrated acids and to reduce the acidity of gastric juice. Also used in buffer solutions.
Application
Sodium bicarbonate (bicarbonate) is used in the chemical, food, light, medical, pharmaceutical industries, non-ferrous metallurgy, and is supplied to retail.
- in the chemical industry - for the production of dyes, foam plastics and other organic products, fluoride reagents, household chemicals, fillers in fire extinguishers, for separating carbon dioxide, hydrogen sulfide from gas mixtures (gas is absorbed in a bicarbonate solution at elevated pressure and low temperature, the solution is reduced at heating and reduced pressure).
- in light industry - in the production of sole rubber and artificial leather, tanning (tanning and neutralizing leather), textile industry (finishing silk and cotton fabrics).
- in the food industry - bakery, confectionery production, preparation of drinks.
Cooking
The main use of baking soda is cooking, where it is used mainly as a main or additional leavening agent
when baking (as it releases carbon dioxide when heated), alone or as part of complex leavening agents (for example, baking powder, mixed with ammonium carbonate), for example, in biscuit and shortbread dough.
Medicine
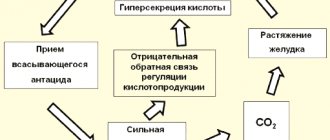
A solution of baking soda is used as a weak antiseptic for rinsing, as well as a traditional acid-neutralizing remedy for heartburn and stomach pain (modern medicine does not recommend its use due to side effects, including “acid rebound”) or to eliminate acidosis, etc.
Firefighting
Sodium bicarbonate is part of the powder used in powder fire extinguishing systems, utilizing heat and displacing oxygen from the combustion source with the released carbon dioxide.
Chemical properties
Sodium bicarbonate is an acidic sodium salt of carbonic acid. Exhibits all the properties of a salt of a strong base and a weak acid. In aqueous solutions it has a slightly alkaline reaction. Over a wide range of concentrations in an aqueous solution, the pH of the solution changes slightly, which is the basis for the use of a solution of the substance as a buffer solution.
Reaction with acids
Sodium bicarbonate reacts with acids to form a salt corresponding to the acid, for example, sodium chloride, sodium sulfate and carbonic acid, which during the reaction breaks down into carbon dioxide and water, while carbon dioxide is released from the solution in the form of bubbles:
NaHCO3 + HCl → NaCl + H2CO3 H2CO3 → H2O + CO2↑ 2NaHCO3 + H2SO4 → Na2SO4 + 2H2O + 2CO2↑
In everyday life, the reaction of “quenching soda” with acetic acid is usually used, with the formation of sodium acetate, or quenching with citric acid with the formation of sodium citrate, reaction with acetic acid:
NaHCO3 + CH3COOH → CH3COONa + H2O + CO2↑
Links
Wikimedia Foundation. 2010.
Useful
See what “Baking soda” is in other dictionaries:
BATTING SODA - sodium bicarbonate NaHCO3; see Soda ... Big Encyclopedic Dictionary
baking soda - sodium bicarbonate, NaHCO3; see Soda. * * * BAKING SODA BAKING SODA, sodium bicarbonate NaHCO3; see Soda (see SODA) ... Encyclopedic Dictionary
Baking soda - sodium bicarbonate NaHCO3, see Soda ... Great Soviet Encyclopedia
BATTING SODA - sodium bicarbonate, NaHCO3; see Soda ... Natural history. encyclopedic Dictionary
baking soda - bicarbonate ... Dictionary of chemical synonyms I
SODA is a name common to several chemical products with alkaline properties: 1) caustic soda, or caustic soda, which is a strong alkali (see Caustic); 2) soda ash and crystalline (or carbon dioxide), called in everyday life... ... Brief encyclopedia of household management
Soda - (Baking soda, sodium bicarbonate, sodium bicarbonate NaHCO3 and sodium carbonate Na2CO3). Modern baking soda is a typical industrial product. However, it was known to mankind long before our era in its natural state... ... Great Encyclopedia of Culinary Arts
Soda - Soda: Soda ash sodium carbonate Na2CO3. Crystalline soda is the general name for crystalline soda hydrates: Soda Na2CO3*10H2O. Thermonatrite Na2CO3*H2O. Baking soda, baking soda, bicarbonate... ... Wikipedia
SODA - SODA: soda ash (sodium carbonate) Na2CO3 (crystals, melting point 858°C) and drinking or baking soda (sodium bicarbonate) NaHCO3. Soda ash is found in nature in the form of minerals, found in underground brines, brine... ...Modern encyclopedia
Content
- 1 Chemical properties 1.1 Reaction with acids
- 4.1 In the chemical industry
Soda: Different names - one essence
Official, systematic name: Sodium bicarbonate
Common, traditional names: Baking soda, baking soda, sodium bicarbonate, sodium bicarbonate.
These are all different “names” of one chemical entity - NaHCO 3 . It is found in its pure form in nature as NAHKOLITE . Remember this name. It is the soda extracted from this mineral that is pure and natural.
Under these names you can find on sale white crystalline powder containing at least 99% sodium bicarbonate. Which is most often called "Baking Soda".
As you probably already understood, the quality of “Baking Soda” directly depends on its composition. And the composition is determined by the level of raw materials, the method and culture of production, the quality of chemicals accompanying the production (or their absence in the case of the water method).
1% impurities (if it is 1%. ) - is it a lot or a little?
1% arsenic in the product? The dose does not seem to be lethal, but it has been proven that when consumed daily, arsenic, even in trace amounts, causes slow poisoning and cancer.
1% aluminum in food? Tiny amounts of aluminum, when consumed regularly, contribute to a variety of mental disorders, including Alzheimer's disease.
What could be included in this 1% in the so-called “Baking Soda”?
According to GOST 2014, the residue may contain arsenic, ammonium salts, aluminum and other impurities.
If you add half a teaspoon of this soda to pancakes once a week, there will most likely be no consequences.
Source
Receipt
In industry, sodium bicarbonate is produced by the ammonia-chloride method. Carbon dioxide is passed under pressure into a concentrated solution of sodium chloride, saturated with ammonia. During the synthesis process, two reactions occur:
NH3 + CO2 + H2O → NH4HCO3 NH4HCO3 + NaCl → NaHCO3↓ + NH4Cl
Sodium bicarbonate is slightly soluble in cold water, and it is separated from the cooled solution by filtration, and from the ammonium chloride solution obtained after filtration, ammonia is again obtained, which is returned to production again:
2NH4Cl + Ca(OH)2 → 2NH3↑ + CaCl2 + 2H2O
Price of Sodium bicarbonate
You can buy sodium bicarbonate in the form of a 4% solution at a pharmacy for 15-28 UAH. In Russian pharmacies its price ranges from 103 to 160 rubles (depending on the volume of the bottle and the manufacturer).
How much does baking soda cost?
The price of baking soda per kg is on average 2.5 UAH (17-25 Russian rubles), for a pack of 500 g - 3.8 UAH (11-16 Russian rubles).
You can buy sodium bicarbonate in bulk for about 3.2-3.5 thousand UAH/t (about 10 thousand rubles/t).
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
ZdravCity
- Sodium bicarbonate solution for inf.
40mg/ml vial. 200ml No. 28 JSC Dalkhimfarm 1880 rub. order
Pharmacy Dialogue
- Sodium bicarbonate (vial 40 mg/ml 200 ml) Dalkhimpharm
RUB 1,727 order
show more
Pharmacy24
- Sodium bicarbonate 4% 100 ml solution TOV "Yuriya-Pharm", Ukraine
27 UAH.order - Sodium bicarbonate 4% 200 ml solution TOV "Yuriya-Pharm", Ukraine
35 UAH order
Reviews
Baking soda is a time-tested remedy “for beauty, cleanliness and health.” These are the reviews about Sodium bicarbonate that can be found on the Internet. It is used for facial cleansing, teeth whitening and caries treatment, for weight loss and hair care, and also for pregnancy detection.
By the way, reviews of baking soda for weight loss are very optimistic, but only in cases where it was considered not as a panacea, but as an adjuvant. Some girls note that after a soda bath they immediately lost up to 2 kg of weight, but without physical activity and nutritional adjustments, the procedures would not have given a visible result.
Alternative medicine uses Sodium Bicarbonate to treat cancer. It is quite difficult to find reviews about soda treatment for oncology describing the results of treatment by the person himself. What is known is that patients of the doctor who promotes this method of treatment also die.
There are no statistics on people cured of cancer using soda. Self-medication when it comes to cancer is fraught with loss of time and the chance of cure.
Side effects
Long-term use of sodium bicarbonate leads to alkalosis (increased blood pH), the clinical manifestations of which are:
- nausea;
- vomit;
- deterioration of appetite (to the point of complete loss);
- stomach ache;
- tetanic convulsions (in especially severe cases);
- increase in blood pressure.
When using suppositories, a laxative effect may develop, the urge to defecate, rumbling, diarrhea and flatulence .
Release form
Powder for preparing a solution for infusion therapy. In bags of 50 g.
Powder for the preparation of a solution for topical use and oral administration. In bags of 10, 25 and 50 g.
Solution for infusion 4%. Available in 2 and 5 ml in disposable containers made of polymer materials; 100, 200 and 400 ml bottles; 100 and 250 ml in polymer containers.
Tablets 0.3 and 0.5 g.
Suppositories for rectal use 0.3, 0.5 and 0.7 g, 10 suppositories per package.
Notes[ | ]
- Glinka N. L.
General chemistry. - M.: “Chemistry”, 1977, revised. - P. 441. - 720 p. - ↑ 1 2 3 4 Mashkovsky M.D.
Medicines (a manual on pharmacotherapy for doctors). - Medicine, 1998. - P. 112. - 688 p. - Rinse for toothache - recommendations
- Rinsing your mouth with baking soda as a remedy for inflammation
- Instructions
- Sodium Bicarbonate (Archive.org copy) // American Cancer Society, 11/28/2008 (English): “Available peer-reviewed medical journals do not support claims that sodium bicarbonate works as a cancer treatment.”
- Chambers, Michael
Sodium bicarbonate [USP:JAN]
(undefined)
.
ChemIDplus
. US National Library of Medicine. - GOST 2156-76 “Sodium bicarbonate. Specifications" Archived January 13, 2010 on the Wayback Machine
Analogs
Synonyms are the drugs Soda Buffer and Sodium bicarbonate . The 4th level ATC code is the same as Glucyl , Potassium chloride , Calcium , Xylate , Lactoxyl , Magnesium sulfate , Sodium chloride , Plerigo , Reamberin .
Thermal decomposition[ | ]
At temperatures above 60 °C, sodium bicarbonate begins to decompose into sodium carbonate, carbon dioxide and water (the decomposition process is most effective at 200 °C:
2 N a HCO 3 → 60 − 200 ∘ CN a 2 CO 3 + H 2 O + CO 2 ↑. {\displaystyle {\mathsf {2NaHCO_{3}{\xrightarrow {60-200^{\circ }C}}Na_{2}CO_{3}+H_{2}O+CO_{2}\uparrow }}. }
During this process, due to the release of carbon dioxide and water (in the form of water vapor), the mass of the original substance decreases by approximately 37%.