Release form and composition
- Chewable tablets (mint): round, flat, with beveled edges, two-layer - one layer is white, the other is pink with darker small inclusions; on one side there is a circle and a sword, on the other - GDA250; tablets have a characteristic mint smell and taste (2, 4, 6 or 8 pieces in blisters; in a cardboard pack there are 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10 blisters);
- suspension for oral administration (mint): an opaque liquid from almost white to light brown in color, has a viscous consistency, a characteristic mint odor [150, 200, 300 or 600 ml each in dark glass bottles, sealed with a polypropylene cap that provides first-opening control ; 10 ml in multilayer (polyester, aluminum, polyethylene) bags, 4, 12 or 24 bags in a cardboard pack].
Each pack also contains instructions for using Gaviscon Double Action.
Composition of 1 tablet:
- active ingredients: sodium alginate – 250 mg; sodium bicarbonate – 106.5 mg; calcium carbonate – 187.5 mg;
- auxiliary components: mannitol, aspartame, macrogol 20,000, acesulfame potassium, copovidone, azorubine dye (11012), xylitol (DC), mint flavor, magnesium stearate.
Composition of 10 ml suspension:
- active ingredients: sodium alginate – 500 mg; sodium bicarbonate – 213 mg; calcium carbonate – 325 mg;
- auxiliary components: sodium saccharinate, carbomer (974P), sodium hydroxide, methyl parahydroxybenzoate, mint flavor, propyl parahydroxybenzoate, purified water.
Gaviscon double action 300ml oral suspension mint
pharmachologic effect
Gaviscon® Double Action is a combination of alginate and antacids (calcium carbonate and sodium bicarbonate).
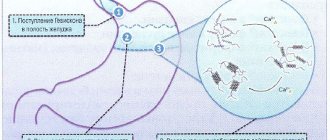
When taken orally, the drug quickly reacts with the acidic contents of the stomach. This forms an alginate gel with an almost neutral pH value. The gel forms a protective shell on the surface of the stomach contents and acts for up to 4 hours, effectively preventing the occurrence of gastroesophageal reflux. In case of regurgitation, the gel enters the esophagus, where it reduces irritation of the mucous membrane. Calcium carbonate quickly neutralizes the hydrochloric acid of gastric juice, relieving the feeling of heartburn. This effect is enhanced by the presence of sodium bicarbonate in the drug, which also has a neutralizing effect.
Composition and release form Gaviscon double action 300ml oral suspension mint
Oral suspension - 10 ml:
- Active ingredients: sodium alginate - 500 mg; sodium bicarbonate - 213 mg; calcium carbonate - 325 mg;
- Excipients: carbomer (974P) - 65 mg, methyl parahydroxybenzoate - 40 mg, propyl parahydroxybenzoate - 6 mg, sodium hydroxide - 26.67 mg, sodium saccharinate - 10 mg, mint flavor - 6 mg, purified water - up to 10 ml.
150/200/300/600 ml - dark glass bottles with first opening control.
10 ml - multilayer bags, cardboard packs.
Description of the dosage form
Oral suspension (mint) is viscous, opaque, almost white to light brown in color, with a minty odor.
Directions for use and doses
The drug is taken orally.
Adults and children over 12 years of age are prescribed 10-20 ml of suspension after meals and before bedtime (up to 4 times a day). The maximum daily dose is 80 ml.
For elderly patients, no dose adjustment is required.
Pharmacokinetics
The mechanism of action of the active substances of the drug does not depend on absorption into the systemic circulation.
Indications for use Gaviscon double action 300ml oral suspension mint
Symptomatic treatment of diseases associated with indigestion, increased acidity of gastric juice and gastroesophageal reflux (heartburn, sour belching), a feeling of heaviness in the stomach, discomfort after eating.
Contraindications
- children under 12 years of age;
- hypersensitivity to any of the components of the drug.
Carefully:
- severe renal dysfunction;
- hypophosphatemia;
- hypercalcemia;
- nephrocalcinosis.
Application of Gaviscon double action 300ml suspension for oral administration mint during pregnancy and breastfeeding
The drug can be used during pregnancy and breastfeeding. The use of the drug is contraindicated in children under 12 years of age.
special instructions
10 ml of suspension contains 127.25 mg (5.53 mmol) sodium. This should be taken into account when it is necessary to follow a diet with limited salt content, for example, in congestive heart failure and impaired renal function.
10 ml of suspension contains 130 mg (3.25 mmol) of calcium. Therefore, caution should be exercised when treating patients with hypercalcemia, nephrocalcinosis, and recurrent calcium-containing renal stones.
Gaviscon® Double Action contains antacids, which can mask the symptoms of serious gastrointestinal diseases. The effectiveness of the drug may be reduced in patients with very low levels of gastric acidity.
Children with gastroenteritis or suspected renal failure are at increased risk of hypernatremia.
The drug should not be used for a long time. If there is no improvement within 7 days, the patient should consult a doctor.
Impact on the ability to drive vehicles and operate machinery
The drug does not affect the ability to drive vehicles and machinery, as well as to engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Overdose
Symptoms: flatulence.
Treatment: symptomatic therapy.
Side effects of Gaviscon double action 300ml oral suspension mint
In rare cases (
Taking calcium carbonate in doses greater than recommended can cause alkalosis, hypercalcemia, milk-alkali syndrome, rebound phenomenon, and constipation.
Drug interactions
The drug contains calcium carbonate, which exhibits antacid activity, therefore, at least 2 hours should elapse between taking Gaviscon® Double Action and other drugs, especially when used simultaneously with histamine H2 receptor blockers, antibiotics from the tetracycline group, digoxin, fluoroquinolones, iron salts, ketoconazole, antipsychotics, levothyroxine sodium, penicillamine, beta-blockers, corticosteroids, chloroquine and diphosphates.
Pharmacological properties
Pharmacodynamics
Gaviscon Double Action is a combined drug for the treatment of reflux esophagitis, including antacids (calcium carbonate and sodium bicarbonate) and alginate.
The mechanism of action of the drug is determined by the properties of its active substances. After oral administration, Gaviscon Double Action quickly reacts with the acidic contents of the stomach. Alginate promotes the formation of a gel with almost neutral acidity, which creates a protective barrier on the surface of the stomach contents, preventing its backflow into the esophagus and the occurrence of gastroesophageal reflux. In severe cases of reflux - regurgitation, the reflux of the gel into the esophagus precedes the reflux of other gastric contents, which reduces irritation of the esophageal mucosa.
Antacids have an alkalizing (acid-neutralizing) effect, which is achieved by taking a minimum dose (2 tablets). The feeling of heartburn and indigestion is relieved by calcium carbonate, which quickly reacts with hydrochloric acid in the gastric juice and neutralizes it. Its combination with sodium bicarbonate enhances the effect.
Pharmacokinetics
After oral administration, the drug is not absorbed into the systemic circulation.
Gaviscon double action TB chew mints N 24
Release form, composition and packaging
Chewable tablets (mint) are round, flat, two-layer, with beveled edges and a mint odor; one layer has pink color and small inclusions of a darker color, the other layer is white; with an image of a circle and a sword on one side of the tablet and the inscription “GDA250” on the other.
| 1 tab. | |
| sodium alginate | 250 mg |
| sodium bicarbonate | 106.5 mg |
| calcium carbonate | 187.5 mg |
Excipients: mannitol - 598.799 mg, macrogol 20,000 - 30 mg, copovidone - 33.75 mg, acesulfame potassium - 5.863 mg, aspartame - 5.863 mg, azorubine dye (11012) - 0.375 mg, mint flavor - 18.75 mg, xylitol (DC) - 100 mg, magnesium stearate - 6.75-12.6 mg.
pharmachologic effect
Antacid drug. When taken orally, the drug quickly reacts with the acidic contents of the stomach. This forms an alginate gel with an almost neutral pH value. The gel forms a protective barrier on the surface of the stomach contents, preventing the occurrence of gastroesophageal reflux. In the case of regurgitation, the gel is more likely to enter the esophagus, where it reduces irritation of the mucous membrane.
Calcium carbonate quickly neutralizes the hydrochloric acid of gastric juice, relieving the sensation of heartburn and indigestion. This effect is enhanced by the presence of sodium bicarbonate in the drug, which also has a neutralizing effect. The total acid-neutralizing activity of the drug in the minimum dose (2 tablets) is approximately 10 mEq.
Pharmacokinetics
The drug does not have systemic bioavailability (not absorbed).
Indications
- treatment of symptoms of gastroesophageal reflux, such as sour belching, heartburn, dyspepsia, a feeling of heaviness in the stomach that occurs after eating, in patients with high acidity of gastric juice or during pregnancy.
Dosage regimen
The drug is taken orally. The tablet should be chewed thoroughly.
Adults and children over 12 years of age are prescribed 2-4 tablets. after meals and before bed (up to 4 times a day).
The maximum daily dose is 16 tablets.
For elderly patients, no dose adjustment is required.
Side effect
The incidence of adverse reactions was assessed based on the following criteria: very often (≥1/10), often (≥1/100, <1/10), infrequently (≥1/1000, <1/100), rarely (≥1 /10,000, <1/1000), very rare (<1/10,000) and frequency unknown (frequency cannot be calculated from available data).
From the immune system: frequency unknown - anaphylactic and anaphylactoid reactions, hypersensitivity reactions (urticaria).
From the respiratory system: frequency unknown - respiratory effects (bronchospasm).
If any of the side effects indicated in the instructions are aggravated or other side effects not listed in the instructions are noted, the patient should inform the doctor.
Contraindications for use
- moderate to severe renal failure;
- phenylketonuria;
- children under 12 years of age;
- hypersensitivity to any of the components of the drug.
The drug should be used with caution in case of hypercalcemia, nephrocalcinosis and urolithiasis with the formation of oxalate stones, congestive heart failure, and mild renal failure. If you have these diseases or conditions, the patient should consult a doctor before using the drug.
Use during pregnancy and breastfeeding
Clinical studies involving more than 500 pregnant women and the amount of data obtained during the post-registration period did not show feto- and neonatal toxicity of the active substances. Gaviscon® Double Action can be used during pregnancy if clinically necessary and after consultation with a physician.
The duration of use of the drug during pregnancy is no more than 7 days.
Gaviscon® Double Action can be used during breastfeeding.
Use for renal impairment
The use of the drug is contraindicated in moderate to severe renal failure.
The drug should be used with caution in case of nephrocalcinosis and urolithiasis with the formation of oxalate stones, mild renal failure.
Use in children
The use of the drug in children under 12 years of age .
Use in elderly patients
For elderly patients, no dose change is required.
special instructions
In a dose of 2 tablets, the sodium content is 110.75 mg (4.82 mmol). This should be taken into account when a salt-restricted diet is required, for example in congestive heart failure and mild renal failure.
Each dose of 2 tablets contains 150 mg (3.75 mmol) calcium. Therefore, caution should be exercised when treating patients with hypercalcemia, nephrocalcinosis and urolithiasis with the formation of oxalate stones.
Due to the content of aspartame, the drug should not be used in patients with phenylketonuria.
Gaviscon® Double Action contains antacids, which can mask the symptoms of serious gastrointestinal diseases.
The effectiveness of the drug may be reduced in patients with low acidity of gastric juice. In patients with gastroenteritis or suspected renal failure, there is an increased risk of hypernatremia when using the drug.
The drug should not be used for a long time. If symptoms persist after 7 days of using the drug, the patient should consult a doctor to review therapy.
Impact on the ability to drive vehicles and operate machinery
The drug does not affect the ability to drive vehicles and machinery, as well as to engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Overdose
Symptoms: bloating.
Treatment: symptomatic therapy.
Drug interactions
The drug contains calcium carbonate, which exhibits antacid activity, therefore, at least 2 hours should elapse between taking Gaviscon®Double Action and other drugs, especially when used simultaneously with histamine H2 receptor blockers, antibiotics from the tetracycline group, digoxin, fluoroquinolones, iron salts, ketoconazole, antipsychotics, levothyroxine sodium, thyroid hormones, penicillamine, beta-blockers (atenolol, metoprolol, propranolol), corticosteroids, chloroquine, bisphosphonates and estramustine.
Storage conditions and periods
The drug should be stored out of the reach of children at a temperature not exceeding 30°C. Shelf life: 2 years.
Conditions for dispensing from pharmacies
The drug is approved for use as a means of OTC.
Contraindications
Absolute:
- age up to 12 years;
- phenylketonuria (for tablets);
- moderate and severe renal failure (for tablets);
- hypersensitivity to Gaviscon components Double action.
With caution, only after consulting a doctor, you can use each of the dosage forms for the following diseases:
- tablets: congestive heart failure, mild renal failure, nephrocalcinosis, urolithiasis (formation of calcium oxalate stones), hypercalcemia;
- suspension: severe renal dysfunction, hypercalcemia, nephrocalcinosis, hypophosphatemia.
Gaviscon Double action, instructions for use: method and dosage
Chewable tablets (mint)
Gaviscon Double Action tablets are taken orally after meals and should be chewed thoroughly before swallowing.
Recommended dosage for patients over 12 years of age: 2–4 pcs. 4 times a day (after each meal and before bedtime).
The maximum daily dose is 16 pcs.
Oral suspension (mint)
Gaviscon Double Action Suspension is taken orally after meals.
Recommended dosage for patients over 12 years of age: 10–20 ml 4 times a day (after each meal and before bedtime).
The maximum daily dose is 80 ml.
Gaviscon (double action chewable tablets No. 12 (mint))
A country
United Kingdom
Country of origin may vary by batch. Please check with the operator for detailed information when confirming your order.
Compound
1 tablet contains: sodium alginate 250 mg, sodium bicarbonate 106.5 mg, calcium carbonate 187.5 mg.
Excipients: mannitol - 598.799 mg, macrogol 20,000 - 30 mg, copovidone - 33.75 mg, acesulfame potassium - 5.863 mg, aspartame - 5.863 mg, azorubine dye (11012) - 0.375 mg, mint flavor - 18.75 mg, xylitol (DC) - 100 mg, magnesium stearate - 6.75-12.6 mg. Chewable tablets (mint) are round, flat, two-layer, with beveled edges and a mint odor; one layer has pink color and small inclusions of a darker color, the other layer is white; with an image of a circle and a sword on one side of the tablet and the inscription “GDA250” on the other.
pharmachologic effect
Antacid drug. When taken orally, the drug quickly reacts with the acidic contents of the stomach. This forms an alginate gel with an almost neutral pH value. The gel forms a protective barrier on the surface of the stomach contents, preventing the occurrence of gastroesophageal reflux. In the case of regurgitation, the gel is more likely to enter the esophagus, where it reduces irritation of the mucous membrane. Calcium carbonate quickly neutralizes the hydrochloric acid of gastric juice, relieving the sensation of heartburn and indigestion. This effect is enhanced by the presence of sodium bicarbonate in the drug, which also has a neutralizing effect. The total acid-neutralizing activity of the drug in the minimum dose (2 tablets) is approximately 10 mEq.
Indications for use
Treatment of symptoms of gastroesophageal reflux, such as sour belching, heartburn, dyspepsia, a feeling of heaviness in the stomach that occurs after eating, in patients with high acidity of gastric juice or during pregnancy.
Mode of application
The drug is taken orally. The tablet should be chewed thoroughly. Adults and children over 12 years of age are prescribed 2-4 tablets. after meals and before bed (up to 4 times a day). The maximum daily dose is 16 tablets. For elderly patients, no dose adjustment is required.
Interaction
The drug contains calcium carbonate, which exhibits antacid activity, therefore, at least 2 hours should elapse between taking Gaviscon® Double Action and other drugs, especially when used simultaneously with histamine H2 receptor blockers, antibiotics from the tetracycline group, digoxin, fluoroquinolones, iron salts, ketoconazole, antipsychotics, levothyroxine sodium, thyroid hormones, penicillamine, beta-blockers (atenolol, metoprolol, propranolol), corticosteroids, chloroquine, bisphosphonates and estramustine.
Side effect
The frequency of adverse reactions was assessed based on the following criteria: very often (?1/10), often (?1/100, From the immune system: frequency unknown - anaphylactic and anaphylactoid reactions, hypersensitivity reactions (urticaria). From the respiratory system : frequency unknown - respiratory effects (bronchospasm).If any of the side effects indicated in the instructions are aggravated or other side effects not listed in the instructions are noted, the patient should inform the doctor.
Contraindications
- moderate to severe renal failure; - phenylketonuria; - children under 12 years of age; - hypersensitivity to any of the components of the drug. The drug should be used with caution in case of hypercalcemia, nephrocalcinosis and urolithiasis with the formation of oxalate stones, congestive heart failure, and mild renal failure. If you have these diseases or conditions, the patient should consult a doctor before using the drug.
Overdose
Symptoms: bloating. Treatment: symptomatic therapy.
special instructions
In a dose of 2 tablets, the sodium content is 110.75 mg (4.82 mmol). This should be taken into account when a salt-restricted diet is required, for example in congestive heart failure and mild renal failure. Each dose of 2 tablets contains 150 mg (3.75 mmol) calcium. Therefore, caution should be exercised when treating patients with hypercalcemia, nephrocalcinosis and urolithiasis with the formation of oxalate stones. Due to the content of aspartame, the drug should not be used in patients with phenylketonuria. Gaviscon® Double Action contains antacids, which can mask the symptoms of serious gastrointestinal diseases. The effectiveness of the drug may be reduced in patients with low acidity of gastric juice. In patients with gastroenteritis or suspected renal failure, there is an increased risk of hypernatremia when using the drug. The drug should not be used for a long time. If symptoms persist after 7 days of using the drug, the patient should consult a doctor to review therapy. Effect on the ability to drive vehicles and operate machinery The drug does not affect the ability to drive vehicles and operate machinery, as well as engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Side effects
Possible adverse reactions due to taking chewable tablets:
- from the immune system: unknown frequency - hypersensitivity reactions, urticaria, anaphylactic and/or anaphylactoid reactions;
- from the respiratory system, chest organs and mediastinum: unknown frequency - bronchospasm.
Possible adverse reactions due to taking the suspension: in rare cases, there is a possibility of developing allergic reactions in the form of bronchospasm, urticaria, anaphylaxis.
special instructions
Patients on a diet with limited salt content should take into account that the amount of sodium in two tablets is 110.75 mg (4.82 mmol), in 10 ml of suspension - 127.25 mg (5.53 mmol).
Due to the presence of calcium in Gaviscon Dual Action, caution should be exercised in patients with hypercalcemia, nephrocalcinosis, urolithiasis with the formation of calcium oxalate stones, or recurrent formation of calcium-containing renal stones. In two tablets the calcium content is 150 mg (3.75 mmol), in 10 ml of suspension - 130 mg (3.25 mmol).
Antacids can mask the symptoms of severe gastrointestinal diseases. In patients with low acidity of gastric juice, the effectiveness of Gaviscon Double action may be reduced.
In case of gastroenteritis or suspected renal failure, taking tablets or suspensions increases the risk of hypernatremia.
If symptoms of malaise persist after 7 days of therapy, it is recommended to consult a doctor. Longer continuous use of the drug is contraindicated.
Impact on the ability to drive vehicles and complex mechanisms
Taking Gaviscon Double Action does not affect the patient's ability to perform potentially hazardous activities that require a high degree of concentration and speed of psychomotor reactions, including driving vehicles and machinery.
Gaviscon double action suspension sachet 10ml No. 12
A country
United Kingdom
Country of origin may vary by batch. Please check with the operator for detailed information when confirming your order.
Compound
Active ingredients: sodium alginate 500 mg, sodium bicarbonate 213 mg, calcium carbonate 325 mg.
pharmachologic effect
This drug is a combination of alginate and antacids (calcium carbonate and sodium bicarbonate). Pharmacodynamics. When taken orally, the drug quickly reacts with the acidic contents of the stomach. This forms an alginate gel with an almost neutral pH value. The gel forms a protective shell on the surface of the stomach contents and acts for up to 4 hours, effectively preventing the occurrence of gastroesophageal reflux. In case of regurgitation, the gel enters the esophagus, where it reduces irritation of the mucous membrane. Calcium carbonate quickly neutralizes the hydrochloric acid of gastric juice, relieving the feeling of heartburn. This effect is enhanced by the presence of sodium bicarbonate in the drug, which also has a neutralizing effect. Pharmacokinetics. The mechanism of action of the active substances of the drug does not depend on absorption into the systemic circulation.
Indications for use
Symptomatic treatment of diseases associated with indigestion, increased acidity of gastric juice and gastroesophageal reflux (heartburn, sour belching), a feeling of heaviness in the stomach, discomfort after eating.
Mode of application
Orally. Adults and children over 12 years of age: 10 - 20 ml after meals and before bedtime (up to 4 times a day). The maximum daily dose is 80 ml. For elderly patients, no dose change is required.
Interaction
Since calcium carbonate, which is part of the drug, exhibits antacid activity, at least 2 hours should pass between taking the drug and other drugs (especially when taking H2-histamine receptor blockers, antibiotics from the tetracycline group, digoxin, fluoroquinolone, iron salts, ketoconazole, antipsychotics, levothyroxine sodium, penicillamine, beta-blockers, glucocorticosteroids, chloroquine and diphosphates).
Side effect
In rare cases, allergic reactions (urticaria, bronchospasm, anaphylactic reactions) are possible. Taking large amounts (more than recommended doses) of calcium carbonate can cause alkalosis, hypercalcemia, milk-alkali syndrome, rebound phenomenon, and constipation.
Contraindications
Increasing sensitivity to any of the components of the drug. Children's age up to 12 years. With caution: severe renal dysfunction; hypophosphatemia; hypercalcemia; nephrocalcinosis. Use during pregnancy and breastfeeding. The drug can be used during pregnancy and breastfeeding.
Overdose
Symptoms: flatulence. Treatment: symptomatic.
special instructions
In 10 ml of suspension the sodium content is 127.25 mg (5.53 mmol). This should be taken into account if it is necessary to follow a diet with limited salt content, for example, with congestive heart failure and impaired night function. 10 ml of suspension contains 130 mg (3.25 mmol) of calcium. Therefore, caution should be exercised when treating patients with hypercalcemia, nephrocalcinosis, and recurrent calcium-containing renal stones. The drug contains antacids, which can mask the symptoms of serious diseases of the gastrointestinal tract. The effectiveness of the drug may be reduced in patients with very low levels of gastric acidity. Children with gastroenteritis or suspected renal failure have an increased risk of hyperpatremia. The drug should not be used for a long time; if there is no improvement within 7 days, you should consult a doctor. Effect on the ability to operate machinery and a car. The drug does not affect the ability to drive vehicles and machinery, as well as to engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.