Pharmacodynamics and pharmacokinetics
The active component of the product is similar in structure to gamma-aminobutyric acid , which is a neurotransmitter . The substance binds to the alpha-2-δ subunit of calcium channels and stops the flow of calcium through them. It is these channels that ensure the transmission of the nerve impulse signaling pain.
The drug also additionally reduces glutamate-dependent neuronal death, increases the synthesis of GABA , and reduces the intensity of the release of monoamine group neurotransmitters .
At normal concentrations in blood plasma, the drug does not affect other GABA receptors , benzodiazepine , glutamate and other receptors. The substance does not interact with sodium channels.
If you increase the dosage of the drug, its bioavailability will, on the contrary, decrease. The maximum concentration in blood occurs 2-3 hours after administration. Bioavailability – about 60%. Eating food, even with a high fat content, does not affect the pharmacokinetic parameters of the drug.
The half-life is about 6 hours. The active substance does not have the ability to bind to blood plasma proteins.
The drug does not accumulate in the body and does not cause induction of liver enzymes . Excretion from the body is carried out through the kidneys with urine. However, in elderly patients and those suffering from liver disease, the clearance of gabapentin is slightly reduced.
Patients with impaired renal function, especially those undergoing periodic hemodialysis , should adjust the daily dosage.
Convalis®
Adverse effects observed in clinical studies of patients with epilepsy (using gabapentin as monotherapy or in combination with other anticonvulsants) or neuropathic pain are presented below and categorized by organ system and frequency.
The frequency category was defined as follows: very often (≥1/10); often (from ≥1/100 to <1/10); uncommon (from ≥1/1000 to <1/100); rare (from ≥1/10000 to <1/1000); very rare (<1/10000). If the frequency category was different between studies, the side effect was assigned a higher category.
Side effects reported during the use of the drug after registration were assigned a frequency category of “unknown” (frequency cannot be calculated based on available data).
In each frequency section, side effects are presented in order of decreasing severity.
Infectious and parasitic diseases:
very often - viral infections;
often - pneumonia, respiratory tract infection, urinary tract infection, other types of infection, otitis media.
Blood and lymphatic system disorders:
often - leukopenia;
unknown - thrombocytopenia.
Immune system disorders:
uncommon - allergic reactions, including urticaria;
unknown - hypersensitivity, including systemic reactions such as fever, rash, hepatitis, lymphadenopathy, eosinophilia and others.
Metabolic and nutritional disorders:
often - anorexia, increased appetite.
Mental disorders:
often - hostility, confusion, depression, anxiety, nervousness, impaired thinking, emotional lability;
infrequently - deterioration of mental state; unknown - hallucinations.
Nervous system disorders:
very often - drowsiness, dizziness, ataxia;
often - convulsions, hyperkinesia, dysarthria, amnesia, tremor, insomnia, headache, sensory disturbances (for example, paresthesia, hypesthesia), loss of coordination, nystagmus, strengthening, weakening or absence of reflexes;
infrequently - hypokinesia; rarely - loss of consciousness;
unknown - other movement disorders (for example, choreoathetosis, dyskinesia and dystonia).
Visual disorders:
often - visual impairment (such as amblyopia, diplopia).
Hearing and labyrinth disorders:
often - vertigo; unknown - tinnitus.
Cardiac disorders:
infrequently - palpitations.
Vascular disorders:
often - symptoms of vasodilation or arterial hypertension.
Disorders of the respiratory system, chest and mediastinal organs:
often - shortness of breath, bronchitis, pharyngitis, cough, rhinitis.
Gastrointestinal disorders:
often - constipation, diarrhea, dry mucous membrane of the oral cavity or pharynx, dyspepsia, flatulence, nausea, vomiting, abdominal pain, dental disease, gingivitis;
unknown - pancreatitis.
Disorders of the liver and biliary tract:
unknown - hepatitis, jaundice.
Disorders of the skin and subcutaneous tissues:
often - swelling of the face, purpura (most often it was described as bruising resulting from physical trauma), skin rash, acne, itching of the skin; unknown - Stevens-Johnson syndrome, angioedema, erythema multiforme, alopecia, drug skin rash, including eosinophilia and systemic reactions (see section "Special instructions").
Musculoskeletal and connective tissue disorders:
often - myalgia, arthralgia, back pain, muscle twitching;
unknown - rhabdomyolysis, myoclonus.
Renal and urinary tract disorders:
unknown - urinary incontinence, acute renal failure.
Disorders of the genital organs and breast:
often - impotence;
unknown - increase in the volume of the mammary glands, gynecomastia, sexual dysfunction (including changes in libido, ejaculation disorders and anorgasmia).
General disorders and disorders at the injection site:
very often - fatigue, fever;
often - peripheral edema, gait disturbance, asthenia, pain of various localizations, general malaise, flu-like syndrome;
infrequently - generalized edema;
unknown - withdrawal syndrome (the most frequently reported side effects were anxiety, insomnia, nausea, pain of various localizations and increased sweating), chest pain.
There is information about cases of sudden unexplained death that coincided with the use of gabapentin. The cause-and-effect relationship of these cases with gabapentin treatment has not been established.
Laboratory and instrumental data:
often - decreased concentration of white blood cells, increased body weight;
infrequently - increased activity of alanine aminotransferase, aspartate aminotransferase and bilirubin concentration in the blood plasma, hyperglycemia;
rarely - hypoglycemia (mainly in patients with diabetes mellitus);
unknown - hyponatremia, increased creatine phosphokinase activity.
Injuries, intoxications and complications of manipulations:
often - injuries, fractures, abrasions associated with falls.
There are reports of the development of acute pancreatitis during gabapentin therapy. The causal relationship with gabapentin remains unclear (see section "Special instructions"). There are reports of cases of myopathy with increased creatine kinase activity in patients with end-stage renal failure undergoing hemodialysis.
Cases of respiratory tract infection, otitis media, bronchitis and seizures were reported only in clinical studies. In addition, clinical studies have reported cases of aggressive behavior and hyperkinesis in children.
If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Contraindications
Capsules cannot be used:
- children under 12 years old;
- for acute pancreatitis ;
- persons with lactose , lactase deficiency and glucose-galactose malabsorption ;
- if you are allergic to the components of the drug.
Patients suffering from kidney failure should be careful when taking the medicine.
Side effects
When using medication to treat neuropathic pain, you may experience:
- ataxia , memory loss, confusion, drowsiness , tremor , dizziness , hyposthesia ;
- diarrhea , constipation , indigestion , excessive gas formation , abdominal pain, dry mucous membranes, nausea;
- skin rashes, amblyopia , shortness of breath ;
- general weakness, headache , asthenia , swelling, weight gain.
If the drug is prescribed for the treatment of partial seizures , the following side effects develop:
- leukopenia , purpura ;
- lability of blood pressure , vasodilation , inflammatory processes in the mouth (diseases of teeth and gums);
- acne , rashes and itching on the skin;
- gingivitis , constipation , diarrhea , increased appetite and indigestion , nausea, flatulence , pain in the epigastric region ;
- back pain, inflammation of muscle tissue, arthralgia , brittle bones;
- dizziness , problems with tendon reflexes, anxiety, depression , depression, nystagmus , thinking disorders;
- runny nose , cough, pneumonia ;
- development of genitourinary ;
- hyperkinesis , paresthesia , amnesia , confusion and impaired motor coordination, dysarthria , insomnia , tremor ;
- decreased libido and impotence ;
- impaired visual acuity, diplopia , fatigue, swelling of the face and periphery, asthenia .
Adverse reactions such as ataxia , dizziness , nystagmus and drowsiness depend on the dose taken.
There have also been reports of the development of acute renal failure , pancreatic , gynecomastia , mastopathy , and hallucinations . In some groups of patients, the following are possible: dystonia , dyskinesia , thrombocytopenia , palpitations, tinnitus, anuria .
If you abruptly stop taking the pills, signs of withdrawal syndrome may occur: nausea, pain in various places, sweating, anxiety, sleep disturbance.
Convalis
In the treatment of neuropathic pain
Gastrointestinal tract:
constipation, diarrhea, dry mouth, dyspepsia, flatulence, nausea, vomiting, abdominal pain.
NS:
gait disturbance, amnesia, ataxia, confusion, dizziness, hypoesthesia, drowsiness, impaired thinking, tremor.
Respiratory system:
shortness of breath, pharyngitis.
Skin:
skin rash.
Sense organs:
amblyopia.
Other:
asthenic syndrome, flu-like syndrome, headache, infectious diseases, pain of various localizations, peripheral edema, weight gain.
In the treatment of partial seizures
SSS:
symptoms of vasodilation, increased blood pressure, increased or decreased blood pressure.
Gastrointestinal tract:
flatulence, anorexia, gingivitis, abdominal pain, constipation, dental disease, diarrhea, dyspepsia, increased appetite, dry mouth or throat, nausea, vomiting.
Blood system, lymphatic system:
purpura (most often described as bruising resulting from physical trauma), leukopenia.
Musculoskeletal system:
arthralgia, back pain, increased bone fragility, myalgia.
NS:
dizziness, hyperkinesis; strengthening, weakening or absence of tendon reflexes, paresthesia, anxiety, hostility, amnesia, ataxia, confusion, incoordination, depression, dysarthria, emotional lability, insomnia, nystagmus, drowsiness, impaired thinking, tremor, muscle fibrillation.
Respiratory system:
pneumonia, cough, pharyngitis, rhinitis.
Skin:
abrasions, acne, itchy skin, skin rash.
Genitourinary system:
urinary tract infection, impotence.
Sense organs:
visual impairment, amblyopia, diplopia.
Other:
asthenic syndrome, facial swelling, fatigue, fever, headache, viral infection, peripheral edema, weight gain.
When comparing the tolerability of the drug at doses of 300 and 3600 mg/day, a dose dependence was noted for such phenomena as dizziness, ataxia, drowsiness, paresthesia and nystagmus.
Post-registration experience of use
Possible cases of sudden unexplained death are not associated with gabapentin treatment. During treatment with gabapentin, the following undesirable effects may be observed: various allergic reactions, acute renal failure, impaired liver function, pancreas, increase in the volume of the mammary glands, gynecomastia, hallucinations, movement disorders (myoclonus, dyskinesia, dystonia), palpitations, thrombocytopenia, noise in the ears, urination disorders. After abrupt discontinuation of gabalentin therapy, the most frequently reported side effects were anxiety, insomnia, nausea, pain of various localizations, and sweating.
If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Instructions for use of Convalis (Method and dosage)
The medicine is taken orally, without chewing or splitting the tablet, regardless of food.
Instructions for use of Convalis as a medicine for epilepsy
The initial dosage is 300 mg per day. Then the daily dose is increased to 900 mg, distributed at regular intervals. Subsequently, if necessary and on the recommendation of the attending physician, the amount of the drug per day can reach 1200 mg.
The maximum amount of gabapentin that can be consumed during the day is 3600 mg (every 8 hours). The interval between doses is no more than 12 hours.
Treatment of neuropathic pain
On the first day, take 300 mg of the drug, on the second - 600 mg in 2 doses, on the third - 300 mg, 3 times a day. Further, the daily dosage can be increased to 3600 mg.
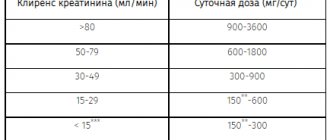
For kidney disease:
- if creatinine clearance is from 50 to 79 ml per minute, then you can drink 600-1800 mg of the drug per day;
- if CC is from 30 to 49 ml/min – up to 900 mg per day;
- if clearance is up to 30 ml/min – 600 mg;
- with clearance less than 15 ml per minute, a dosage of 300 mg per day should be followed.
In patients undergoing hemodialysis , an additional 300 mg of the drug should be taken after each 4-hour session.
On days when dialysis is not performed, increasing the daily dose is not advisable.
Convalis, 50 pcs., 300 mg, capsules
Treatment of neuropathic pain
From the gastrointestinal tract:
constipation, diarrhea, dry mouth, dyspepsia, flatulence, nausea, vomiting, abdominal pain.
From the nervous system:
gait disturbance, amnesia, ataxia, confusion, dizziness, hypoesthesia, drowsiness, impaired thinking, tremor.
From the respiratory system:
shortness of breath, pharyngitis.
From the skin:
skin rash.
From the senses:
amblyopia.
Other:
asthenic syndrome, flu-like syndrome, headache, infectious diseases, pain of various localizations, peripheral edema, weight gain.
Treatment of partial seizures
From the SSS side:
symptoms of vasodilation, increased or decreased blood pressure.
From the gastrointestinal tract:
flatulence, anorexia, gingivitis, abdominal pain, constipation, dental disease, diarrhea, dyspepsia, increased appetite, dry mouth or throat, nausea, vomiting.
From the blood system, lymphatic system:
purpura (most often in the form of bruises resulting from physical trauma), leukopenia.
From the musculoskeletal system:
arthralgia, back pain, increased bone fragility, myalgia.
From the nervous system:
dizziness, hyperkinesis; strengthening, weakening or absence of tendon reflexes, paresthesia, anxiety, hostility, amnesia, ataxia, confusion, incoordination, depression, dysarthria, emotional lability, insomnia, nystagmus, drowsiness, impaired thinking, tremor, muscle fibrillation.
From the respiratory system:
pneumonia, cough, pharyngitis, rhinitis.
From the skin:
acne, skin itching, skin rash.
From the genitourinary system:
urinary tract infection, impotence.
From the senses:
visual impairment, amblyopia, diplopia.
Other:
asthenic syndrome, facial swelling, fatigue, fever, headache, viral infection, peripheral edema, weight gain.
When comparing the tolerability of the drug at doses of 300 and 3600 mg/day, a dose dependence was noted for such phenomena as dizziness, ataxia, drowsiness, paresthesia and nystagmus.
Post-registration experience of use
Possible cases of sudden unexplained death are not associated with gabapentin treatment.
During treatment with gabapentin, the following undesirable effects may be observed: various allergic reactions, acute renal failure, impaired liver function, pancreas, enlargement of the mammary glands, gynecomastia, hallucinations, movement disorders (myoclonus, dyskinesia, dystonia), palpitations, thrombocytopenia, noise in the ears, urination disorders.
After abrupt discontinuation of gabapentin therapy, the most frequently reported side effects were anxiety, insomnia, nausea, pain of various localizations, and sweating.
If any of the side effects indicated in the instructions worsen or the patient notices any other side effects not listed in the instructions, the doctor should be informed.
Interaction
Cimetidine increases the period of elimination of gabapentin from the body.
Concomitant use of the drug with oral contraceptives containing ethinyl estradiol or norethisterone does not cause drug interactions.
The combination of the drug with morphine , if morphine was taken 120 minutes before gabapentin , leads to a prolongation of the AUC of the drug by 50% and an increase in pain.
When taking the drug simultaneously with other anticonvulsants ( Phenobarbital, Valproic acid, Carbamazepine, Phenytoin ), no interaction between the drugs occurs.
Antacids that contain aluminum or magnesium reduce the bioavailability of the drug. These medications should be taken at intervals of 2 hours.
When combining the drug with ethanol, side effects may increase.
Co-administration with Naproxen leads to an increase in the absorption time of Convalis.
special instructions
Sometimes, after starting to take the drug for people suffering from diabetes , dosage adjustment of hypoglycemic agents .
When analyzing urine for protein content using litmus paper , the results may be distorted. It is recommended to carry out analyzes using any other method.
It is not recommended to drive a car or perform activities requiring high concentration while you are taking Convalis.
pancreatitis is recognized during drug therapy , treatment must be interrupted.
Discontinuation or replacement of tablets should be done gradually over 7 days due to the increased risk of seizures.
It is also recommended to carry out timely monitoring of the patient’s mental state. During therapy, the risk of developing depression and suicidal thoughts and actions increases.
Convalis (Gabapentin) (caps. 300 mg No. 50)
A country
Russia
The country of production may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Active substance
Gabapentin
Compound
1 capsule contains: gabapentin 300 mg.
Excipients: lactose monohydrate - 66 mg, pregelatinized corn starch - 30 mg, talc - 3 mg, magnesium stearate - 1 mg. Composition of the capsule body and cap: titanium dioxide (E171) - 2%, iron dye yellow oxide (E172) - 0.6286%, gelatin - up to 100%. Capsules size No. 0, yellow. The contents of the capsules are white or yellowish crystalline powder.
pharmachologic effect
Gabapentin is structurally similar to the neurotransmitter GABA, but its mechanism of action differs from that of some other drugs that interact with GABA receptors, including valproic acid, barbiturates, benzodiazepines, GABA transaminase inhibitors, GABA uptake inhibitors, GABA agonists and GABA prodrugs: it does not has GABAergic properties and does not affect the uptake and metabolism of GABA. Preliminary studies suggest that gabapentin binds to the α2-δ subunit of voltage-gated calcium channels and inhibits the flow of calcium ions, which plays an important role in neuropathic pain. Other mechanisms involved in the action of gabapentin in neuropathic pain are: a decrease in glutamate-dependent neuronal death, an increase in GABA synthesis, and a suppression of the release of monoamine neurotransmitters. Gabapentin at clinically significant concentrations does not bind to other receptors, including GABAA, GABAB, benzodiazepine, glutamate, glycine, or N-methyl-d-aspartate receptors. Unlike phenytoin and carbamazepine, gabapentin does not interact with sodium channels in vitro. Gabapentin partially attenuated the effects of the glutamate receptor agonist N-methyl-d-aspartate in some in vitro tests, but only at concentrations greater than 100 μmol/L, which are not achieved in vivo. Gabapentin slightly reduces the release of monoamine neurotransmitters in vitro.
Indications for use
- epilepsy: in adults and children over 12 years of age - as monotherapy or as part of combination therapy for the treatment of partial epileptic seizures, incl. occurring with secondary generalization; - for the treatment of neuropathic pain in adults.
Side effects
In the treatment of neuropathic pain From the digestive system: constipation, diarrhea, dry mouth, dyspepsia, flatulence, nausea, vomiting, abdominal pain. From the side of the central nervous system: gait disturbance, amnesia, ataxia, confusion, dizziness, hypoesthesia, drowsiness, impaired thinking, tremor. From the respiratory system: shortness of breath, pharyngitis. From the skin: skin rash. From the senses: amblyopia. Other: asthenic syndrome, flu-like syndrome, headache, infectious diseases, pain of various localizations, peripheral edema, weight gain. In the treatment of partial seizures From the cardiovascular system: symptoms of vasodilation, increased or decreased blood pressure. From the digestive system: flatulence, anorexia, gingivitis, abdominal pain, constipation, dental disease, diarrhea, dyspepsia, increased appetite, dry mouth or pharynx, nausea, vomiting. From the blood system: purpura (most often it was described as bruising that occurs during physical trauma), leukopenia. From the musculoskeletal system: arthralgia, back pain, increased bone fragility, myalgia. From the side of the central nervous system: - dizziness, hyperkinesis; - strengthening, weakening or absence of tendon reflexes, paresthesia, anxiety, hostility, amnesia, ataxia, confusion, impaired coordination of movements, depression, dysarthria, emotional lability, insomnia, nystagmus, drowsiness, impaired thinking, tremor, muscle fibrillation. From the respiratory system: pneumonia, cough, pharyngitis, rhinitis. From the skin: abrasions, acne, skin itching, skin rash. From the urinary system: urinary tract infection. From the reproductive system: impotence. From the senses: visual impairment, amblyopia, diplopia. Other: asthenic syndrome, facial swelling, fatigue, fever, headache, viral infection, peripheral edema, weight gain. When comparing the tolerability of the drug at doses of 300 and 3600 mg/day, a dose dependence was noted for such phenomena as dizziness, ataxia, drowsiness, paresthesia and nystagmus. Post-registration experience Possible cases of sudden unexplained death are not associated with treatment with gabapentin. During treatment with gabapentin, the following undesirable phenomena may be observed: various allergic reactions, acute renal failure, impaired liver function, pancreas, increased volume of the mammary glands, gynecomastia, hallucinations, movement disorders (myoclonus, dyskinesia, dystonia), palpitations, thrombocytopenia, noise in ears, urinary disorders. After abrupt discontinuation of gabapentin therapy, the most frequently reported side effects were anxiety, insomnia, nausea, pain of various localizations, and sweating. If any of the side effects indicated in the instructions are aggravated or other side effects not listed in the instructions are noted, you must inform your doctor.
Contraindications
- acute pancreatitis;
- children under 12 years of age; - lactase deficiency, lactose intolerance, glucose-galactose malabsorption; - hypersensitivity to the drug and/or its components. Use during pregnancy and lactation There is no sufficient data on the use of gabapentin in pregnant women. The drug should not be used during pregnancy unless the potential benefit to the mother outweighs the possible risk to the fetus. The drug passes into breast milk, the effect on children during breastfeeding is unknown, therefore, during breastfeeding, the drug should be used only in cases where the potential benefit to the mother from taking the drug clearly outweighs the potential risk to the infant.
Use in children The use of the drug is contraindicated in children under 12 years of age.
Mode of application
The drug is taken orally, regardless of food intake, without chewing and with the required amount of liquid. Monotherapy and the use of Convalis as an adjuvant for the treatment of partial epileptic seizures in children over 12 years of age and adults Treatment begins with a dose of 300 mg 1 time / day and is gradually increased to 900 mg / day (the first day - 300 mg 1 time / day, the second - 300 mg 2 times/day, third - 300 mg 3 times/day). Subsequently, the dose may be increased. Typically, the dose of Convalis is 900-1200 mg/day. The maximum dose is 3600 mg/day, divided into three equal doses every 8 hours. The maximum interval between doses of the drug should not exceed 12 hours to avoid resumption of seizures. Neuropathic pain in adults Treatment begins with a dose of 300 mg on the first day, then: 600 mg (300 mg 2 times) on the second day, 900 mg (300 mg 3 times) on the third day. For intense pain, Convalis® can be used from the first day at 300 mg 3 times a day. Depending on the effect, the dose can be gradually increased, but not more than 3600 mg/day. In patients with impaired renal function (with CC 50-79 ml/min), the daily dose of the drug is 600-1800 mg/day, with CC 30-49 ml/min - 300-900 mg/day, with CC 15-29 ml/day min - 300-600 mg/day, with CC less than 15 ml/min - 300 mg every other day or daily. In patients on hemodialysis, the initial dose of Convalis is 300 mg. The additional post-hemodialysis dose is 300 mg after each 4-hour hemodialysis session. On days when dialysis is not performed, Convalis® is not used.
special instructions
When testing urine for total protein using the Ames N-Multistix SG® Test System, a false positive result is possible. It is necessary to confirm the obtained result using another method of analysis. In patients with diabetes mellitus, there is sometimes a need to change the dose of hypoglycemic drugs. If signs of acute pancreatitis appear, treatment with the drug should be stopped. The drug should be discontinued or replaced with an alternative remedy gradually, over at least a week. Abrupt cessation of anticonvulsant therapy in patients with partial seizures may provoke the development of seizures. There may be an increased risk of suicide and suicidal thoughts. In order to early identify behavioral disorders that may be precursors of suicidal thoughts and actions, it is recommended to monitor the mental state of patients. Impact on the ability to drive vehicles and operate machinery During the treatment period, it is necessary to refrain from driving vehicles and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Overdose
Symptoms: with a single dose of 49 g of gabapentin, dizziness, diplopia, speech impairment, drowsiness, dysarthria, and diarrhea were observed. The lethal dose of gabapentin when taken orally was established in mice and rats receiving the drug in doses of 8000 mg/kg. Signs of acute toxicity in animals included ataxia, labored breathing, ptosis, hypoactivity or agitation. Treatment: symptomatic therapy. Hemodialysis may be indicated for patients with severe renal failure.
Interaction with other drugs
When gabapentin and morphine were coadministered, when morphine was taken 2 hours before gabapeptin, there was a 44% increase in mean gabapentin AUC compared to gabapentin monotherapy, which was associated with an increase in pain threshold (cold pressor test). The clinical significance of this change has not been established; the pharmacokinetic characteristics of morphine did not change. The side effects of morphine when taken together with gabapentin did not differ from those when morphine was taken together with placebo. No interactions were observed between gabapentin and phenobarbital, phenytoin, valproic acid and carbamazepine. The pharmacokinetics of gabapentin at steady state are similar in healthy subjects and patients receiving other anticonvulsants. Concomitant use of gabapentin with oral contraceptives containing norethisterone and/or ethinyl estradiol was not accompanied by changes in the pharmacokinetics of both components. Antacids containing aluminum or magnesium reduce the bioavailability of gabapentin by approximately 20%. In this regard, the drug should be taken no earlier than 2 hours after taking antacids. Pimetidine slightly reduces the renal excretion of gabapentin. Ethanol and agents acting on the central nervous system may increase the central nervous system side effects of gabapentin. When naproxen is administered simultaneously with gabapentin, the absorption of the latter increases, while gabapentin does not affect the pharmacokinetic parameters of naproxen. The simultaneous use of gabapentin with hydrocodone leads to a decrease in the pharmacokinetic parameters (Cmax and AUC) of hydrocodone and an increase in the AUC of gabapentin.
Dispensing conditions in pharmacies
On prescription
Convalis analogs
Level 4 ATC code matches:
Gabagamma
Neurontin
Pagluferal
Algerica
Topiramate
Lamotrigine
Lamictal
Gabapentin
Topamax
Keppra
Pregabalin Richter
Tebantin
The most common analogues of the drug: Algerica, Gerolamic, Lamictal, Normeg, Lamitril, Latrigyl, Topiramin, levetiracetam, Levitsam, Lyrica, Epimil, Topilex, Neogabine, Topamax, Topilepsin, Epileptal, Epiramat, Epitrizhin, Vimpat, Keppra, Lamitor, Lamotrin, Carbamazepine .
Reviews about Convalis
The medicine is reviewed well, mainly in the treatment of epilepsy . The most common adverse reactions are headaches, nausea and digestive problems. They also complain about the occurrence of withdrawal syndrome when abruptly stopping taking the pills.
Reviews about Convalis on the forums:
- “... A very good drug, however, you need to take it for a long time and adhere to the prescribed treatment regimen”;
- “... I suffer from a herniated disc, but on Convalis I felt much better, I could walk a lot, I was more active. But after finishing the course, the pain returned”;
- “... Neuropathic pain remains at the same level. True, after 2-3 hours after taking it, I manage to fall asleep for 3-4 hours.”
Convalis
In the treatment of neuropathic pain
From the digestive system: constipation, diarrhea, dry mouth, dyspepsia, flatulence, nausea, vomiting, abdominal pain.
From the side of the central nervous system: gait disturbance, amnesia, ataxia, confusion, dizziness, hypoesthesia, drowsiness, impaired thinking, tremor.
From the respiratory system: shortness of breath, pharyngitis.
From the skin: skin rash.
From the senses: amblyopia.
Other: asthenic syndrome, flu-like syndrome, headache, infectious diseases, pain of various localizations, peripheral edema, weight gain.
In the treatment of partial seizures
From the cardiovascular system: symptoms of vasodilation, increased or decreased blood pressure.
From the digestive system: flatulence, anorexia, gingivitis, abdominal pain, constipation, dental disease, diarrhea, dyspepsia, increased appetite, dry mouth or pharynx, nausea, vomiting.
From the blood system: purpura (most often it was described as bruising that occurs during physical trauma), leukopenia.
From the musculoskeletal system: arthralgia, back pain, increased bone fragility, myalgia.
From the side of the central nervous system: dizziness, hyperkinesis; strengthening, weakening or absence of tendon reflexes, paresthesia, anxiety, hostility, amnesia, ataxia, confusion, incoordination, depression, dysarthria, emotional lability, insomnia, nystagmus, drowsiness, impaired thinking, tremor, muscle fibrillation.
From the respiratory system: pneumonia, cough, pharyngitis, rhinitis.
From the skin: abrasions, acne, skin itching, skin rash.
From the urinary system: urinary tract infection.
From the reproductive system: impotence.
From the senses: visual impairment, amblyopia, diplopia.
Other: asthenic syndrome, facial swelling, fatigue, fever, headache, viral infection, peripheral edema, weight gain.
When comparing the tolerability of the drug at doses of 300 and 3600 mg/day, a dose dependence was noted for such phenomena as dizziness, ataxia, drowsiness, paresthesia and nystagmus.
Post-registration experience of use
Possible cases of sudden unexplained death are not associated with gabapentin treatment. During treatment with gabapentin, the following undesirable phenomena may be observed: various allergic reactions, acute renal failure, impaired liver function, pancreas, increased volume of the mammary glands, gynecomastia, hallucinations, movement disorders (myoclonus, dyskinesia, dystonia), palpitations, thrombocytopenia, noise in ears, urinary disorders.
After abrupt discontinuation of gabapentin therapy, the most frequently reported side effects were anxiety, insomnia, nausea, pain of various localizations, and sweating.
If any of the side effects indicated in the instructions are aggravated or other side effects not listed in the instructions are noted, you must inform your doctor.