International Classification of Diseases (ICD-10)
C16 Malignant neoplasm of the stomach C17 Malignant neoplasm of the small intestine C18 Malignant neoplasm of the colon C19 Malignant neoplasm of the rectosigmoid junction C20 Malignant neoplasm of the rectum C34 Malignant neoplasm of the bronchi and lung C43 Malignant melanoma of the skin C64 Malignant neoplasm of the kidney, except the renal pelvis C71 Malignant neoplasm of the brain brain C81 Hodgkin's disease [lymphogranulomatosis] C90.0 Multiple myeloma
Note!
Description of the drug Lomustine Medak caps. 40 mg No. 20 on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.
Contraindications
- hypersensitivity to lomustine, other nitrosourea derivatives or components of the drug;
- pregnancy and breastfeeding.
Carefully:
myelosuppression (including against the background of concomitant radiation or chemotherapy, intoxication); chicken pox (including recently suffered or after contact with sick people), herpes zoster and other acute infectious diseases of a viral, fungal or bacterial nature; cachexia, intoxication, renal and/or liver failure, respiratory failure, history of treatment with cytostatics and radiation therapy.
Overdose
Accidental overdose with Lomustine, including death, has been reported. In case of overdose, the following side effects should be expected: myelotoxicity, toxic effects on the hematopoietic system, abdominal pain, diarrhea, nausea, vomiting, anorexia, lethargy, dizziness, liver dysfunction, cough, difficulty breathing, gastrointestinal and neurological disorders disorders.
In cases of overdose, gastric lavage is recommended.
There is no special antidote for an overdose of Lomustine. Symptomatic or supportive therapy should be used. According to clinical indications, it is necessary to compensate for the loss of blood cells.
Side effects
From the hematopoietic organs:
thrombocytopenia develops after 4 weeks, leukopenia 5-6 weeks after using the drug and can last 1-2 weeks. Thrombocytopenia is usually more severe than leukopenia. Anemia and granulocytopenia are less common.
Lomustine may cause cumulative myelosuppression, with greater bone marrow suppression occurring after repeated doses or the duration of myelosuppression being longer.
From the digestive organs:
nausea and vomiting (3-6 hours after taking lomustine, usually lasting up to 24 hours), anorexia. The frequency and duration of these side effects can be reduced through the prophylactic use of antiemetic drugs, as well as by administering lomustine to patients on an empty stomach. Rarely - diarrhea, stomatitis, increased activity of liver enzymes and bilirubin concentration.
From the respiratory system:
rarely - cough, respiratory failure, accompanying the appearance of infiltrates and/or fibrosis of the lungs (noted 6 months or longer after the start of treatment at total doses of the drug more than 1100 mg/m2. One case of pulmonary toxicity was reported at a cumulative dose of 600 mg/m2 ).
From the nervous system:
disorientation, lethargy, ataxia, speech articulation disorder, increased fatigue.
From the urinary system:
urinary retention, swelling of the feet or lower extremities, azotemia, reduction in kidney size (usually with high cumulative doses of the drug during long-term treatment with lomustine and other nitrosourea drugs).
From the reproductive system:
azoospermia (irreversible in some cases), amenorrhea.
Other:
rarely - alopecia, irreversible damage to the optic nerves, leading to blindness (when combined with radiation therapy to the brain). Acute leukemias and bone marrow dysplasias have been reported as a result of treatment with nitrosoureas.
LOMUSTINE
The frequency of adverse reactions listed below is presented in accordance with the following gradation: very often (>1/10), often (>1/100, ≤1/10), infrequently (>1/1000, ≤1/100), rarely (>1/10000, ≤1/1000), very rare (≤1/10000), frequency unknown (cannot be determined based on available data).
Disorders of the blood and lymphatic system: very often: suppression of bone marrow hematopoiesis, thrombocytopenia, leukopenia, anemia.
Benign, malignant and unspecified neoplasms (including cysts and polyps): frequency unknown: the development of acute leukemia and bone marrow dysplasia has been reported in patients receiving long-term nitrosourea preparations.
Gastrointestinal disorders: very common: nausea, vomiting, anorexia; rarely: diarrhea, stomatitis.
Disorders of the liver and biliary tract: often: impaired liver function (in most cases mild); rarely: cholestatic jaundice, liver failure.
Disorders of the respiratory system, chest and mediastinal organs: rarely: interstitial pneumonia, infiltrative processes, pulmonary fibrosis.
Nervous system disorders: rarely: impaired coordination, confusion, drowsiness, apathy, speech articulation disorder, stuttering (the development of these symptoms has been reported during combination therapy with other anticancer drugs and radiation therapy).
Renal and urinary tract disorders: frequency unknown: renal failure, progressive azotemia, decreased size (atrophy) of the kidneys.
Skin and subcutaneous tissue disorders: rare: alopecia.
Violation of the organ of vision: very rarely: damage to the optic nerves (in combination with radiation therapy for brain tumors), irreversible loss of vision.
Impact on the results of laboratory and instrumental studies: frequency unknown: increased activity of liver enzymes (aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase), increased bilirubin concentration in the blood serum.
The main undesirable side effect of lomustine is delayed or prolonged myelotoxicity, which usually occurs 4-6 weeks after dosing and is dose-dependent. Thrombocytopenia occurs approximately 4 weeks after a dose of lomustine and usually persists at 80,000 to 100,000/mcL for 1 to 2 weeks. After 5-6 weeks, leukopenia appears (about 4000-5000/μl), which persists for one or two weeks. Anemia is less common, however, compared to thrombocytopenia and leukopenia, it is observed less frequently and is less severe.
Hematologic toxicity may be cumulative, with greater bone marrow suppression occurring after repeated doses. In approximately 65% of patients receiving the drug at a dose of 130 mg/m2, the concentration of leukocytes decreased to levels less than 5000/μl. In 36% of patients this figure was less than 3000/μl. As a rule, thrombocytopenia is more severe than leukopenia; however, both types of toxicity may be dose-limiting.
Nausea and vomiting usually appear 4-6 hours after administration of a full single dose of lomustine and last 24-48 hours, anorexia usually lasts for 2-3 days. The severity of these side effects can be reduced by dividing a single 6-week dose into three doses on the first three days of each 6-week cycle. The drug is usually well tolerated if its use is accompanied by prophylactic antiemetics (for example, metoclopramide or chlorpromazine).
In isolated cases, after long-term treatment with lomustine and other nitrosourea drugs, when a high cumulative dose was reached, renal failure, progressive azotemia, and a decrease in the size (atrophy) of the kidneys were observed. In this regard, it is recommended not to exceed the maximum total cumulative dose of lomustine 1000 mg/m2. However, it must be taken into account that kidney damage is also possible in patients who received lower cumulative doses of the drug.
Mode of application
Lomustine should be taken orally in the evening, before bedtime or 3 hours after meals. The recommended dose of lomustine in adults and children is 130 mg/m2 given once orally every 6 weeks.
In patients with reduced bone marrow function, the dose can be reduced to 100 mg/m2 while maintaining a six-week interval between doses.
In the case of combination therapy, the drug is used at a dose of 70-100 mg/m2.
Repeated courses should not be prescribed if the platelet count is less than 100,000/µl and the leukocyte count is less than 4,000/µl.
The total dose for all courses of treatment should not exceed 1000 mg/m2.
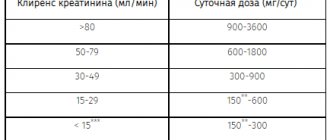
Further doses of the drug should be selected depending on the patient's hematological response to the previous dose. When selecting doses, you can focus on the following scheme:
| Minimum values after taking the previous dose | Recommended next dose | |
| Leukocytes/µl | Platelets/µl | (% of previous dose) |
| 3000-4000 | 75000- 100000 | 100% |
| 2000 – 2999 | 25000 – 74999 | 70% |
| less than 2000 | less than 25000 | 50% |
Features of application
Lomustine Medak is prescribed by oncologists who have experience in the use of anticancer drugs.
The most common and severe toxic effect of Lomustine is late suppression of bone marrow function, namely, a significant decrease in the number of leukocytes and platelets in the blood, as a result of which bleeding and generalized infections occur in patients with reduced immunity.
Therefore, before using the first dose of the drug and then frequently (preferably weekly for at least 6 weeks after the start of treatment), it is necessary to carry out a blood cell analysis.
The dosage regimen for Lomustine Medak is determined solely by the doctor and depends on such blood parameters as the level of hemoglobin, leukocytes and platelets.
When using Lomustine Medak, it is necessary to periodically check the functional state of the liver, kidneys and lungs.
Patients should be warned not to exceed the dose of Lomustine recommended by their physician, and Lomustine should be administered as a single dose every 6 weeks and not repeated for at least 6 weeks.
Pregnant
The safety of the drug during pregnancy has not been established. Therefore, when using the drug during pregnancy or if pregnancy occurs while taking Lomustine, the patient should be warned about the potential risk to the fetus.
Children
Treatment of oncological diseases (except brain tumors) with Lomustine Medak should be carried out only in specialized centers and in exceptional situations. The dose for children, as for adults, depends on body surface area (120-130 mg/m2 every 6-8 weeks) and is adjusted according to the same criteria.
Drivers
Considering that patients may experience adverse reactions when using the drug, while taking the drug you should refrain from driving vehicles and performing other work that requires concentration.
A comment
The work is devoted to an obviously urgent topic - the treatment of patients with glioblastomas of the brain. The advantage of the work is the analysis of one’s own results of treating patients in routine practice, careful collection of follow-up records, thoughtful analysis of the results obtained, and the use of data from not only foreign, but also domestic studies when discussing them. Information was collected on 107 patients who were treated at the Research Institute of Neurosurgery named after. N.N. Burdenko in 2010-2012 Given the high cost, treatment of glioblastomas in the Russian Federation in a significant number of cases does not meet international standards. A large number of patients treated in one institution in full compliance with the standards is a rarity, however, as shown in the work, it is this approach that allows not only to reproduce, but also to exceed the results of treatment in the world's best clinics. From a practical point of view, the data obtained indicate that there is no need to increase the number of courses of chemotherapy with temozolomide to more than six are important.
A.V. Smolin (Moscow)
There is no conflict of interest.
Side effects
- Benign neoplasms, malignant and unspecified cysts (including polyps). Unknown: acute leukemia, myelodysplastic syndrome.
- From the blood side. Very common: leukopenia; unknown: bone marrow failure, thrombocytopenia, anemia.
- From the nervous system. Unknown: coordination problems, disorientation, lethargy, dysarthria.
- From the respiratory system. Unknown: pulmonary fibrosis, pulmonary infiltration.
- From the gastrointestinal tract. Unknown: nausea, vomiting, stomatitis.
- From the digestive system. Unknown: increased transminase, increased bilirubin in the blood.
- From the skin and subcutaneous tissue. Unknown: alopecia.
- From the kidneys and urinary tract. Not known: renal failure, azotemia, renal atrophy, renal damage.
Lomustin
Description of the active substance (INN):
Lomustin
Dosage form:
capsules
Pharmachologic effect:
An antitumor drug with an alkylating effect (from the nitrosourea group). In cells it is cleaved to form methylcarbonium ions, which act on the nucleophilic centers of DNA, RNA, proteins and alkylate their molecules; damages translation and transcription in tumor cells. Inhibition of DNA synthesis is caused by carbamoylation of DNA polymerase and other DNA repair enzymes and damage to the DNA matrix. Causes a significant number of chromosomal aberrations in tumor cells and bone marrow, the correction of which does not coincide in time in normal and tumor cells (the rate of reduction of chromosomal aberrations in tumor cells is much lower than in healthy ones, and damage to the genetic apparatus is more profound; disturbances in the kinetics and cell proliferation and cell cycle are more pronounced in tumor tissues). The effect on the phases of the cell cycle is expressed in a slowdown in the passage of S phase by cells (at a time when DNA synthesis is significantly inhibited). The passage of the G2 phase is lengthened. The highest sensitivity to lomustine is in cells in the stationary growth phase (a factor that determines activity in solid tumors with a low proliferative pool).
Indications:
Neoplasms of the central nervous system, metastatic brain tumors, tumors of the pharynx, larynx, bronchi, lungs, lymphogranulomatosis, lymphosarcoma, malignant lymphoma, myeloma, plasmacytoma, stomach cancer, colon cancer, disseminated melanoma of the skin.
Contraindications:
Hypersensitivity, pregnancy, lactation period. With caution. Suppression of bone marrow function (including against the background of concomitant radiation or chemotherapy, intoxication), acute infectious diseases of a viral, fungal or bacterial nature (including chicken pox, herpes zoster), cachexia, renal and/or liver failure , respiratory failure.
Side effects:
Leukopenia, thrombocytopenia, rarely - anemia; nausea, vomiting, loss of appetite, stomatitis, diarrhea, alopecia, liver dysfunction, amenorrhea, azoospermia, infiltrates and pulmonary fibrosis. Overdose. Symptoms: increased severity of side effects. Treatment: symptomatic.
Directions for use and dosage:
Orally, at a dose of 100-130 mg/sq.m once, with an interval of 6 weeks, or 75 mg/sq.m with an interval of 3 weeks. When combined with other cytostatics, the dose of lomustine is from 70 to 100 mg/sq.m with an interval of 6 weeks.
Special instructions:
Due to some “lag” in the appearance of signs of myelotoxicity, other cytostatic drugs can be prescribed no earlier than 3-6 weeks from the date of drug administration. Do not open the capsules, since the powder contained in them is irritating; contact of the powder with the skin and mucous membranes should be avoided. The increased risk of pulmonary toxicity should be taken into account when used in patients with broncho-obstructive syndrome. Vaccination of patients and their family members is not recommended during therapy with lomustine. Systematic (at least once a week) monitoring of the peripheral blood picture is necessary during therapy and for 6 weeks after the end of treatment (taking into account the likelihood of a late onset of myelotoxic effect), as well as periodic monitoring of laboratory parameters of liver and kidney function.
Interaction:
When used simultaneously with drugs that inhibit hematopoiesis, myelotoxicity may increase.