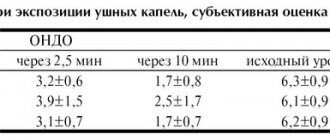
Registration number:
P N011568/01
Trade name
Otipax®
International nonproprietary or generic name
Lidocaine + phenazone
Dosage form
Ear drops
Compound
1 g of solution contains: Active ingredients: lidocaine hydrochloride 10 mg, phenazone 40 mg; Excipients: sodium thiosulfate 1 mg, ethanol 221.8 mg, glycerol 709 mg, water 18.2 mg.
Description
A clear, colorless or yellowish solution with the odor of alcohol.
Pharmacotherapeutic group
Local anti-inflammatory, analgesic antiseptic. ATX code: S02DA30
Pharmacological properties
Pharmacodynamics
Phenazone has anti-inflammatory and analgesic effects. Lidocaine has a local anesthetic effect.
Pharmacokinetics
The drug does not penetrate the body if the eardrum is intact.
Indications for use
Local symptomatic treatment and pain relief in children from birth and adults with otitis media with an intact eardrum, including:
- acute exudative otitis media;
- otitis as a complication after influenza, exudative viral otitis;
- barotraumatic otitis
Contraindications
- Hypersensitivity to the components of the drug;
- Perforation of the eardrum (including those of infectious or traumatic origin)
Carefully
It is necessary to ensure the integrity of the eardrum before starting to use the drug. If the drug is used with a perforated eardrum, the drug may come into contact with the organs of the middle ear and lead to complications.
Use during pregnancy and breastfeeding
If the eardrum is intact and the drug is used correctly, the likelihood of active substances entering the systemic circulation is extremely low. In this regard, in the absence of contraindications, the use of Otipax® by pregnant women and women during breastfeeding is possible.
Directions for use and doses
Otipax® is used in children from birth and adults topically, by instilling 4 drops into the external auditory canal on the affected side 2-3 times a day. To avoid contact of the cold solution with the auricle, the bottle should be warmed in the palms before use. The duration of treatment with Otipax® is no more than 10 days, after which the prescribed treatment should be reconsidered.
Side effect
There is a risk of local allergic reactions, irritation and hyperemia of the ear canal. Please notify your doctor of all cases of undesirable reactions not listed in the instructions.
Overdose
No cases of drug overdose have been reported.
Interaction with other drugs
There is no information about clinically significant interactions with other drugs and (or) foods.
special instructions
In order to prevent complications, before starting the use of the drug Otipax®, it is recommended to consult an otolaryngologist to exclude perforation of the eardrum. If the symptoms of the disease worsen or there is no improvement, a second consultation with a doctor is necessary after 2-3 days of treatment. When using the drug for the first time or resuming treatment, no special actions are required. There are no additional or special actions when skipping one or more doses of the drug, or when discontinuing it. Athletes must take into account that the drug Otipax® contains an active substance that can give a positive result during doping control. No special precautions are required when disposing of unused drugs.
Impact on the ability to drive vehicles and machinery
Otipax® does not affect the ability to drive vehicles and machines.
Release form
Ear drops, 10 mg/g + 40 mg/g. 16 g each in an amber glass bottle with a screw cap and a dropper, packed in a PVC/paper blister, placed in a cardboard box with attached instructions for medical use. It is possible to have a first opening control.
Best before date
3 years. After opening the bottle, the drug should be used within 1 month. Do not use after the expiration date stated on the package.
Storage conditions
At a temperature not exceeding 30 oC. Keep out of the reach of children!
Vacation conditions
Available without a prescription.
Manufacturer:
Biocode, France Registered address: 7, Avenue Gallienie, 94250 Gentilly, France
Manufacturer's address:
1, Avenue Blaise Pascal, 60000 Beauvais, France.
You can obtain additional information about the drug, as well as send your complaints and information about adverse events to the following address in Russia:
LLC "BIOCODEX" 119049, Moscow, per. Yakimansky, 6, building 1 Tel. Fax E-mail Internet address: www.biocodex.ru
Otipax ear drops (40mg+10mg)/1g 16g No.1
Name
Otipax.
Release form
Ear drops.
Dosage
16 g. Quantity per package: 1 pc.
Manufacturer
Laboratory biocode.
INN
Lidocaine + phenazone.
FTG
Anti-inflammatory agent.
Compound
1 g of solution contains: Active substances: lidocaine hydrochloride 10 mg; phenazone 40 mg; Excipients: Sodium thiosulfate, ethanol, glycerol, water.
Description
A clear, colorless or yellowish solution with the odor of alcohol.
Pharmacotherapeutic group
Means used in otology. Analgesics and anesthetics. ATX code.
S02DA30
Pharmacological properties
Phenazone has anti-inflammatory and analgesic effects. Lidocaine has a local analgesic effect. The drug does not penetrate the body if the eardrum is intact.
Indications
Local symptomatic treatment of painful conditions of the middle ear, provided that the integrity of the eardrum is preserved: acute otitis media with congestion; otitis media, as a complication after the flu; barotraumatic otitis media.
Contraindications
hypersensitivity to the components of the drug. perforation of the eardrum. Carefully. Do not use Otipax® and consult a doctor: if you have any discharge from the ear; in case of traumatic damage to the eardrum; with perforation of the eardrum of any nature; with chronic purulent otitis media; with a sharp decrease in hearing, which may be a sign of perforation of the eardrum.
Pregnancy and lactation
There are no restrictions on the use of the drug during pregnancy and breastfeeding, provided that the eardrum is intact.
Side effect
There is a risk of allergic reactions, irritation and hyperemia of the ear canal. If the drug is used with a perforated eardrum, the drug may come into contact with the structures of the middle ear and lead to complications. Information for athletes: the drug contains an active component that can give a positive reaction during doping control.
Directions for use and doses
Otipax® is applied topically by instilling 3-4 drops into the external auditory canal 2-3 times a day. To avoid contact of the cold solution with the eardrum, the bottle should be warmed in the palms before use. The duration of use of the drug Otipax® is no more than 10 days, after which the doctor should reconsider the prescribed treatment. After opening the bottle for the first time, the drug should be used within 6 months. Do not use after the expiration date indicated on the package. Use in children. Otipax® is indicated for use in children from birth 2-3 times a day, 3-4 drops into the external auditory canal.
Interaction with other drugs
Currently, there is no information on interactions with other drugs.
Impact on the ability to drive vehicles and operate machinery
Absent.
Release form
Ear drops 16 g in an amber glass bottle with a screw cap with a dropper, packed in a PVC/paper blister, placed in a cardboard box with attached instructions for medical use.
Best before date
5 years.
Storage conditions
Store at a temperature not exceeding 30° C. Keep out of the reach of children.
Conditions for dispensing from pharmacies
Over the counter.
Buy Otipax ear drops (40mg+10mg)/1g 16g No. 1 in the pharmacy
Price for Otipax ear drops (40mg+10mg)/1g 16g No. 1
Instructions for use for Otipax ear drops (40mg+10mg)/1g 16g No. 1
Precautionary measures
According to the instructions, the drug is contraindicated if you are intolerant of its components (especially to lidocaine). In addition, the drug should not be used after mechanical injury to the eardrum. During lactation, the medication is not contraindicated.
Otipax is well tolerated by patients, but sometimes negative reactions occur in the form of itching, redness or a slight burning sensation. An allergic reaction occurs in the area of the ear where the droplets settle. There is no information on cases of drug overdose.
Doctors remind that drops are allowed to be used only after the patient is convinced that there is no damage to the eardrum and ear canal. We are talking not only about wounds, but also about scratches and abrasions. If a patient applies drops to a damaged ear canal, the likelihood of inflammation, pain and complications increases.
Otipax
Otipax is a combination drug that has anti-inflammatory and local analgesic effects and is used in otolaryngological practice for local symptomatic (aimed at eliminating the symptoms, not the cause of the disease) treatment of otitis media in adults and children. Due to its highly favorable safety profile, the drug is widely used in pediatrics, incl. and in newborn children. If in the theater everything starts with a hanger, then problems with the ears almost always begin with ARVI. This statement is especially relevant in relation to children, because the latter are much more susceptible to infectious agents than adults. ARVI pathogens enter the body primarily through the upper respiratory tract. Penetrating into the epithelial cells of the mucous membranes of the nasal cavity, pharynx and larynx, respiratory viruses are mistaken for intensive reproduction of their own kind, resulting in the development of inflammation with all the accompanying “bonuses” in the form of increased permeability of vascular walls and increased exudation. Clinically, all this pathogenetic “whirlwind” is manifested by cough, runny nose, swelling of the mucous membranes and other dubious “charms”. The most common symptom of ARVI is definitely a runny nose. With a runny nose, swelling of the mucous membrane of the nasopharynx develops, which can lead to acute otitis media. The danger of this disease is the risk of serious complications - mastoiditis, meningeal syndrome, meningitis, labyrinthitis, brain abscess and, possibly, sepsis. In this regard, the problem of otitis takes on a medical and social character, i.e.
because inflammation of the middle ear is always fraught with hearing disorders and has all the prerequisites for transition to the chronic phase in the form of adhesive and purulent otitis. With timely and adequate treatment, these unpleasant consequences can be avoided. So, in case of acute otitis, as a rule, the problem can be solved without resorting to surgical methods, for example, using Otipax ear drops. This medicine contains phenazone and lidocaine. The analgesic-antipyretic phenazone has anti-inflammatory and analgesic effects. Lidocaine is a substance in demand in cases where local anesthesia is necessary. The combination of phenazone and lidocaine provides fast, intense and long-lasting anesthesia. The components of the drug act locally and are not absorbed into the systemic circulation, which avoids clinically significant negative side reactions. When otipax is applied topically, its active components and their derivatives are not detected by modern laboratory diagnostic methods in the blood and other biological media, which confirms the absence of a systemic effect on the body. The dosage regimen for otipax is as follows: 3-4 drops into the external auditory canal 2-3 times a day. Before using the drug, in order to avoid unpleasant sensations from contact of cold drops with the auricle, it is recommended to warm the bottle in the palms of your hands. The duration of treatment should not exceed 10 days. Before starting drug therapy, you should ensure the integrity of the eardrum. This is done for this purpose. to avoid complications due to contact of pharmacologically active substances of the drug with elements of the middle ear system.
Otipax during lactation
Sometimes lactating women suffer from otitis media. The disease is manifested by congestion, discomfort, pain in the ear, fever, etc. If such symptoms appear, it is recommended to contact an otolaryngologist, who will conduct an examination and prescribe the appropriate medication.
Women are wondering whether a nursing mother can use Otipax to treat otitis media. According to the instructions, the drug is allowed to be used for hepatitis B to treat ear inflammation. Doctors say that the components of the drug do not penetrate into the bloodstream or breast milk. That is, the drug does not have a systemic effect, for this reason it is prescribed to pregnant, lactating women, as well as infants.
Since the medicine does not penetrate the baby’s body, nursing mothers do not have to stop lactation during treatment. The drops are safe for infants if they are not allergic to the components.
Thus, Otipax during breastfeeding is an effective and safe drug that quickly relieves pain and inflammation.
Before taking the medication, you should consult a doctor who will prescribe the dosage. To avoid negative reactions, it is necessary to follow the recommendations of the otolaryngologist. Subscribe to our VKontakte group
Brief overview of the drug
Otipax is produced in the form of ear drops, which contain the following substances:
- phenazone;
- lidocaine hydrochloride;
- sodium thiosulfate;
- ethanol (95%);
- glycerol;
- distilled water.
In appearance, it is a clear or yellowish liquid with the aroma of alcohol. The drops are in dark glass bottles with a special dropper included.
Otipax is often used in otolaryngology, as the local drug eliminates the inflammatory process and relieves pain. The effect of the medication is due to its constituent components:
- Phenazone is a non-steroidal anti-inflammatory drug, as well as an analgesic drug with an anti-inflammatory effect. This component inhibits cyclooxygenase (an enzyme that promotes the synthesis of prostanoids), inhibits the production of prostaglandins, as a result, inflammation, swelling, and pain disappear.
- Lidocaine is a local anesthetic component. Under the influence of the substance, the pain impulse is no longer carried out; this occurs due to antagonism with sodium and calcium of the axon membrane (nerve fiber).
Thanks to these components, a pain-relieving effect appears that lasts for a long time. After using the drops, the mucus is liquefied and removed from the middle ear. If the eardrum is not damaged, the drug does not penetrate into the bloodstream.
Otipax is prescribed for the following diseases:
- Otitis of the middle or external ear with an acute course, which is accompanied by severe pain.
- Ear damage due to barotrauma.
- Ear inflammation as a complication after influenza or acute respiratory diseases.
Before using a topical medication, you should consult your doctor.
Application and dosage
The bottle is closed with a sealed cap, which you need to unscrew, remove the foil, and then put on a special dispenser. Close the top of the bottle with a cap and place it in a cool, dark place.
Shake the bottle before use to evenly distribute the particles. The bottle is turned over and the medicine is dripped into the sore ear. The daily dosage of the drug is 4 drops three times. The therapeutic course lasts about 10 days; if necessary, the doctor can extend the treatment.
Before treating the ear, it is recommended to slightly warm the drops. To do this, they are warmed in the palms or held over steam for several seconds.