Home | About us | Delivery | Advertisers | Login | Registration
Delivery on Sundays and holidays does not work!
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2022 Pharmacy 84.
Dexilant caps. 60 mg No. 28 (dexlansoprazole)
Full description
a:2:{s:4:"TEXT";s:77598:"[RU]
Tradename
Dexilant™
International nonproprietary name
Dexlansoprazole
Dosage form
Modified release capsules 30 mg and 60 mg
Composition
One capsule contains
active ingredients: dexlansoprazole 30 mg or 60 mg
excipients: granulated sugar, magnesium carbonate, sucrose, low-substituted hyprolose, hyprolose, hypromellose 2910, talc, titanium dioxide (E 171), methacrylic acid copolymer dispersion, macrogol 8000, polysorbate 80, colloidal anhydrous silicon dioxide, methacrylic acid copolymer (type B), methacrylic acid copolymer (type A), triethyl citrate
Capsule shell:
Carrageenan, potassium chloride, titanium dioxide (E 171), FD&C blue dye
No. 2 aluminum varnish (E132), black iron oxide (E 172), hypromellose, purified gray ink
— composition of granulated sugar: sucrose, corn starch;
** - composition of methacrylic acid copolymer dispersion: methacrylic acid, ethyl acrylate, sodium lauryl sulfate, polysorbate;
*** — composition of purified gray ink: red iron oxide (E 172), yellow iron oxide (E 172), FD&C blue dye No. 2, aluminum varnish (E 132), carnauba wax, shellac, glycerol monooleate.
Description
Capsules with an opaque blue cap and an opaque gray body. The “TAR” logo is applied to the cap in dark gray ink, and the inscription “30” is printed on the body. The contents of the capsules are white to almost white granules
(for a dosage of 30 mg)
Capsules with opaque blue cap and body. The “TAR” logo is applied to the cap in dark gray ink, and the inscription “60” is printed on the body. The contents of the capsules are white to almost white granules (for a dosage of 60 mg)
Pharmacotherapeutic group
Drugs for the treatment of diseases associated with acidity disorders. Antiulcer drugs and drugs for the treatment of gastroesophageal reflux. Proton pump inhibitors. Dexlansoprazole.
ATX code A02BC06
Pharmacological properties
Pharmacokinetics
Dexilant™ is a dual sustained release drug that results in two different peak plasma concentrations; the first peak occurs within one to two hours after ingestion, followed by a second peak within four to five hours. The half-life of dexlansoprazole is about one to two hours in healthy people and in patients with symptoms of gastroesophageal reflux disease (GERD). There was no accumulation of dexlansoprazole after multiple or single doses of Dexilant™ 30 mg or 60 mg, although the mean area under the curve (AUCt) and maximum drug concentration achieved in plasma (Cmax) of dexlansoprazole were slightly higher (less than 10%) at 5 -th day than on the 1st day.
The pharmacokinetics of dexlansoprazole are highly variable, with coefficient of variation (CV%) values for Cmax, AUC, and oral clearance (CL/F) greater than 30% percent (see Table 1).
| Table 1: Average values (CV%) of pharmacokinetic parameters in patients on day 5 after taking Dexilant TM | |||
| Dose (mg) | Cmax (ng/ml) | AUC24 (ng h/ml) | CL/F (l/h) |
| 30 | 658 (40%) (N=44) | 3275 (47%) (N=43) | 11,4 (48%) (N=43) |
| 60 | 1397 (51%) (N=79) | 6529 (60%) (N=73) | 11,6 (46%) (N=41) |
Absorption
Following oral administration of 30 mg or 60 mg of Dexilant™ to healthy individuals and patients with symptomatic GERD, the mean Cmax and AUC of dexlansoprazole increased approximately proportionally to the dose.
When Dexilant™ 60 mg granules are mixed with water and administered through a nasogastric tube or orally syringed, the bioavailability (Cmax and AUC) of dexlansoprazole is similar to that of Dexilant™ 60 mg administered as an unopened capsule.
Distribution
Plasma protein binding of dexlansoprazole in healthy volunteers ranged from 96% to 99% and was independent at concentrations of 0.01-20 μg/L. In patients with symptomatic gastroesophageal reflux disease, the apparent volume of distribution (Vz/F) after multiple doses was 40 L.
Metabolism
Dexlansoprazole is extensively metabolized in the liver by oxidation, reduction and subsequent formation of sulfate, glucuronide and glutathione compounds to inactive metabolites. Oxidative metabolites are formed through the cytochrome P450 (CYP) enzyme system, including hydroxylation, mainly by CYP2C19, and oxidation to sulfone by CYP3A4.
The CYP2C19 isoenzyme is a polymorphic hepatic isoenzyme that exhibits three phenotypes when metabolizing CYP2C19 substrates; rapid metabolizers (*1/*1), intermediate metabolizers (*1/mutant) and poor metabolizers (mutant/mutant). Dexlansoprazole is the major component circulating in plasma, regardless of CYP2C19 metabolizer status. In intermediate and rapid metabolizers of the CYP2C19 isoenzyme, the main metabolite in the blood plasma is 5-hydroxydexlansoprazole and its glucuronic compound, while in slow metabolizers of CYP2C19 the sulfone is the main metabolite in the blood plasma.
Selection
After administration of Dexilant™, dexlansoprazole is not excreted unchanged in the urine. After administration of 14C-labeled dexlansoprazole to six healthy volunteers, approximately 50.7% (standard deviation (SD): 9.0%) of the administered radioactivity was excreted in the urine and 47.6% (SD: 7.3%) in the feces. Oral clearance in healthy individuals was 11.4-11.6 l/h, respectively, after 5 days of use at doses of 30 mg or 60 mg once daily.
Impact of food intake
In meal studies with healthy volunteers, taking Dexilant™ after a meal was compared with taking the drug on an empty stomach; the increase in Cmax ranged from 12% to 55%, the increase in AUC ranged from 9% to 37%, and Tmax also varied (ranging from a decrease of up to 0.7 hours to an increase of up to three hours).
Special patient groups
Childhood
The pharmacokinetics of dexlansoprazole in patients under 18 years of age have not been studied.
Elderly age
In the elderly, compared with the young, the terminal half-life is significantly longer (2.2 and 1.5 hours, respectively). Systemic exposure (AUC) of dexlansoprazole in elderly patients was significantly higher than in younger patients (34% higher).
Floor
When a single 60 mg oral dose of Dexilant™ was administered to 12 healthy men and 12 healthy women, women had a higher systemic exposure (AUC) (43% higher) than men. This difference does not pose a significant safety concern.
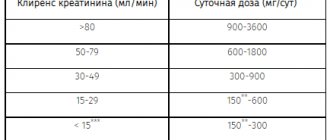
Kidney failure
Dexlansoprazole is extensively metabolized in the liver to inactive metabolites, and the parent substance is not detectable in urine after oral administration of dexlansoprazole. Therefore, the pharmacokinetics of dexlansoprazole is not expected to change in patients with impaired renal function. No studies have been conducted in patients with impaired renal function. In addition, the pharmacokinetics of lansoprazole were not clinically different in patients with mild, moderate or severe renal impairment compared with healthy subjects with normal renal function.
Liver dysfunction
In 12 patients with moderate hepatic impairment (Child-Pugh B) who received a single 60 mg oral dose of Dexilant™, the systemic exposure (AUC) of bound and unbound dexlansoprazole was approximately twice that of patients with normal liver function. liver function. This difference in exposure was not due to differences in protein binding. Studies have not been conducted in patients with severe liver dysfunction.
Pharmacodynamics
Mechanism of action
Dexlansoprazole belongs to the class of antisecretory drugs, is a benzimidazole derivative that suppresses the secretion of gastric juice by inhibiting H+/K+-ATPase in the parietal cells of the stomach. Since this enzyme is considered to be the acid (-proton) pump in the parietal cells, dexlansoprazole is characterized as an inhibitor of the gastric proton pump, since it blocks the final stage of acid formation.
Antisecretory activity
In a crossover study in healthy subjects, the antisecretory effect on 24-hour intragastric pH was assessed when Dexilant™ 60 mg (n = 20) and lansoprazole 30 mg (n = 23) were administered once daily for five days. The results are shown in Table 2.
| Table 2. Effect on 24-hour intragastric pH on day 5 of Dexilant™ and lansoprazole | |
| Dexilant™ 60 mg | Lansoprazole 30 mg |
| Mean intragastric pH | |
| 4.55 | 4.13 |
| Time period with intragastric pH level >4, % (hours) | |
| 71 (17 hours) | 60 (14 hours) |
Effect on serum gastrin levels
The effect of Dexilant™ on serum gastrin concentrations was assessed in approximately 3460 patients in clinical studies lasting up to eight weeks, and in 1023 patients lasting six to 12 months. The mean fasting gastrin concentration increased compared to the control group during treatment with Dexilant™ 30 mg and 60 mg. In patients treated for more than six months, mean serum gastrin levels increased during approximately the first three months of treatment and were stable for the remainder of treatment. Mean serum gastrin levels returned to pre-treatment levels within one month of drug cessation.
Increased gastrin causes hyperplasia of enterochromaffin-like cells (ECL) and serum chromogranin A (CgA) levels. Elevated CgA levels may cause false-positive results in diagnostic tests for neuroendocrine tumors.
Effect on enterochromaffin-like cells (ECL)
When using Dexilant™ 30 mg, 60 mg or 90 mg for up to 12 months, there were no reports of ECL cell hyperplasia based on gastrobiopsy specimens. When lansoprazole was administered to rats up to 150 mg/kg/day throughout life, the development of hypergastrinemia was observed, followed by proliferation of ECL cells and the formation of carcinoid tumors, especially in females.
Effect on cardiac repolarization
At doses up to five times the maximum recommended dosage, dexlansoprazole does not prolong the QT interval to any clinically significant extent.
Indications for use
- treatment of erosive esophagitis of any severity;
- maintenance therapy of treated erosive esophagitis and relief of heartburn;
- for the treatment of heartburn associated with symptomatic nonerosive gastroesophageal reflux disease (GERD)
Directions for use and doses
Important information for use
- Missed Doses: If a dose is missed, take the capsule as soon as possible. However, if the next scheduled dose is already due, do not take the missed dose, but take the next dose on time. Do not take two doses at the same time to make up for a missed dose.
- Dexilant™ can be taken with or without food.
- Swallow whole; do not chew.
- For patients with swallowing problems, Dexilant™ capsules may be opened and taken with applesauce as follows:
1. Place one tablespoon of applesauce in a clean container.
2. Open the capsule.
3. Sprinkle granules into applesauce.
4. Immediately swallow the applesauce granules. Do not chew the granules. Do not store applesauce granules for later use.
- Alternatively, the capsules may be administered with water, an oral syringe, or a nasogastric tube.
Administration with water using an oral syringe
1. Open the capsule and pour the granules into a clean container with 20 ml of water.
2. Place the entire mixture into the syringe.
3. Gently mix the contents of the syringe to prevent the granules from settling.
4. Immediately put the mixture into your mouth. Do not store the mixture of water and granules for later use.
5. Refill the syringe with 10 ml of water, shake gently and insert into the mouth.
6. Refill the syringe again with 10 ml of water, shake gently and insert into the mouth.
Administration with water through a nasogastric tube (>16 French gauge)
1. Open the capsule and pour the granules into a clean container with 20 ml of water.
2. Place the entire mixture into a syringe with a catheter at the end.
7. Gently mix the contents of the syringe to prevent sedimentation of the granules and immediately inject the mixture into the stomach through a nasogastric tube. Do not store the mixture of water and granules for later use.
3. Refill the syringe with 10 ml of water, shake gently and pour into the probe.
4. Refill the syringe with 10 ml of water, shake gently and inject into the stomach.
Dexilant™ is available as capsules in 30 mg and 60 mg doses. Dosage recommendations for adults are given in Table 3.
| Table 3. Dexilant™ Dosing Recommendations for Adults | ||
| Indication | Recommended dose | Frequency |
| Treatment of erosive esophagitis | 60 mg | Once a day, up to 8 weeks |
| Maintenance therapy of treated erosive esophagitis and relief of heartburn symptoms | 30 mg | Once a day*, up to 6 months |
| Symptomatic non-erosive GERD | 30 mg | Once a day, up to 4 weeks |
*Controlled studies lasted no more than 6 months
Dose adjustment in adult patients with erosive esophagitis and liver dysfunction
In patients with moderate hepatic impairment (Child-Pugh class B), the daily dose should not exceed 30 mg of dexlansoprazole once daily for up to 8 weeks.
The use of Dexilant™ is not recommended in patients with severe liver dysfunction (Child-Pugh class C).
Side effects
The following serious adverse reactions are described below and elsewhere in the instructions:
- Acute interstitial nephritis
- Cyanocobalamin (vitamin B12) deficiency
- Clostridial diarrhea
- Bone fracture
- Hypomagnesemia
Clinical research experience
Because clinical trials are conducted under different conditions, the proportion of adverse reactions observed during trials cannot be directly compared with the proportion of reactions in clinical trials with another drug. In addition, they may not reflect the frequency found in practice.
The safety of Dexilant™ was assessed in 4,548 patients in controlled and uncontrolled clinical studies, including 863 patients treated for at least six months and 203 patients treated for one year. The studies included patients aged 18 to 90 years (mean age 48 years), 54% women, 85% Caucasian, 8% black, 4% Asian, and 3% other races. Six randomized controlled clinical trials were conducted on the treatment of erosive esophagitis, maintenance therapy of treated erosive esophagitis and symptomatic GERD. They included 896 patients receiving placebo, 455 patients receiving Dexilant™ 30 mg, 2218 patients receiving 60 mg and 1363 patients receiving lansoprazole 30 mg once daily.
The most common (at least 2%) unwanted side effects are diarrhea, flatulence, abdominal pain, nausea, vomiting, and upper respiratory tract infections.
Adverse reactions leading to discontinuation of therapy
In controlled clinical studies, the most common reaction leading to discontinuation of Dexilant™ was diarrhea (0.7%).
Less frequent adverse reactions
Other adverse reactions reported in controlled studies with an incidence of less than 2% are listed below by body system:
Circulatory and lymphatic system disorders: anemia, lymphadenopathy
Cardiac disorders: angina pectoris, arrhythmia, bradycardia, chest pain, edema, myocardial infarction, palpitations, tachycardia
Hearing and balance disorders: ear pain, tinnitus, dizziness
Endocrine system disorders: goiter
Eye disorders: eye irritation, eye swelling
Gastrointestinal Disorders: Abdominal discomfort, abdominal tenderness, abnormal bowel movements, anal discomfort, Barrett's esophagus, bezoar, abnormal bowel sounds, breath odor, microscopic colitis, colon polyp, constipation, dry mouth , duodenitis, dyspepsia, dysphagia, enteritis, belching, esophagitis, gastric polyp, gastritis, gastroenteritis, gastrointestinal disorders, gastrointestinal hyperkinetic disorders, GERD, gastrointestinal ulcers and perforation, hematemesis, bloody stools, hemorrhoids, impaired gastric emptying, syndrome irritable bowel disease, mucous stools, blistering of the oral mucosa, painful bowel movements, proctitis, oral paresthesia, rectal hemorrhage, retching
General and administration site conditions: adverse drug reaction, asthenia, chest pain, chills, abnormal sense of touch, inflammation, mucosal inflammation, nodular thickening, pain, hyperthermia
Disorders of the liver and biliary tract: biliary colic, cholelithiasis, hepatomegaly
Immune system disorders: hypersensitivity
Infectious and parasitic diseases: candidiasis, influenza, nasopharyngitis, oral herpes, pharyngitis, sinusitis, viral infection, vulvo-vaginal infections
Injuries, poisoning and complications of procedures: falls, fractures, joint dislocations, overdose, pain during the procedure, sunburn
Laboratory tests: increased alkaline phosphatase, increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), increased/decreased bilirubin, increased blood creatinine, increased blood gastrin, increased blood glucose, increased blood potassium, abnormal liver function tests , decreased platelet count, increased total protein, weight gain
Metabolic and nutritional disorders: changes in appetite, hypercalcemia, hypokalemia
Skeletal muscle and connective tissue disorders: arthralgia, arthritis, muscle cramps, musculoskeletal pain, myalgia
Nervous system disorders: changes in taste, convulsions, dizziness, headaches, migraines, memory impairment, paresthesia, psychomotor hyperactivity, tremor, trigeminal neuralgia
Mental disorders: unusual dreams, anxiety, depression, insomnia, changes in libido
Renal and urinary tract disorders: dysuria, urinary urgency
Reproductive system and breast disorders: dysmenorrhea, dyspareunia, menorrhagia, menstrual irregularities
Respiratory, thoracic and mediastinal disorders: aspiration, asthma, bronchitis, cough, dyspnea, hiccups, hyperventilation, airway congestion, sore throat
Skin and subcutaneous tissue disorders: acne, dermatitis, erythema, itching, rash, skin lesions, urticaria
Cardiovascular system disorders: deep vein thrombosis, hot flashes, hypertension
Additional adverse reactions reported in a long-term, uncontrolled study and considered by the treating physician to be related to Dexilant™ included: anaphylaxis, auditory hallucinations, B-cell lymphoma, bursitis, central obesity, acute cholecystitis, dehydration, diabetes mellitus, dysphonia, epistaxis, folliculitis, gout, herpes zoster, hyperlipidemia, hypothyroidism, increased number of neutrophils, decreased average hemoglobin concentration in erythrocytes, neutropenia, painful urge to defecate, restless leg syndrome, drowsiness, tonsillitis.
Post-registration experience
The following adverse reactions were identified during post-registration use of the drug Dexilant™. Because these reactions have been reported voluntarily from the public to an unknown extent, it is not always possible to reliably estimate the frequency of their occurrence or establish a causal relationship to drug exposure.
Circulatory and lymphatic system disorders: autoimmune hemolytic anemia, idiopathic thrombocytopenic purpura
Hearing and balance disorders: deafness
Visual disorders: blurred vision
Gastrointestinal disorders: oral edema, pancreatitis
General and injection site conditions: facial swelling
Liver and biliary tract disorders: drug-induced hepatitis
Immune system disorders: anaphylactic shock (requiring emergency intervention), exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis (sometimes fatal)
Infectious and parasitic diseases: clostridial diarrhea
Metabolic and nutritional disorders: hypomagnesemia, hyponatremia
Musculoskeletal disorders: bone fracture
Nervous system disorders: cerebrovascular accident, transient ischemic attack
Renal and urinary tract disorders: acute renal failure
Respiratory, thoracic and mediastinal disorders: laryngeal edema, throat constriction
Skin and subcutaneous tissue disorders: generalized rash, leukocytoclastic vasculitis
Contraindications
- hypersensitivity to any of the components of the drug
- combined use with drugs containing rilpivirine
- acute interstitial nephritis
- children under 18 years of age
Drug interactions
Tables 4 and 5 describe clinically significant drug interactions with Dexilant™, interactions between diagnostic tests and Dexilant™, and recommendations for preventing complications.
| Table 4. Clinically significant drug interactions with Dexilant™ and interactions with diagnostic tests | ||
| Antiretroviral drugs | ||
| Clinical impact: | The effect of proton pump inhibitors (PPIs) on antiretroviral drugs is variable. The clinical significance and mechanisms underlying these interactions are not always known.
| |
| Prevention measures: | Products containing rilpivirine: use with Dexilant™ is contraindicated. Atazanavir: See atazanavir prescribing information for dosing information. Nelfinavir: Avoid use with Dexilant™. See instructions for use of nelfinavir. Saquinavir: See saquinavir prescribing information and monitor for potential saquinavir toxicity. Other antiretroviral drugs: see instructions for use. | |
| Warfarin | ||
| Clinical impact: | Increased international normalized ratio (INR) and prothrombin time in patients receiving PPIs and warfarin simultaneously. An increase in INR and prothrombin time can lead to bleeding and even death. | |
| Prevention measures: | Monitoring INR and prothrombin time. Warfarin dosage adjustments may be necessary to maintain the target INR range. See instructions for use of warfarin. | |
| Methotrexate | ||
| Clinical impact: | Concomitant use of PPIs with methotrexate (usually at high doses) may increase levels and prolong the presence of serum methotrexate and/or its metabolite hydroxymethotraxate, which may lead to methotrexate toxicity. No formal studies have been conducted on the interaction of high-dose methotrexate with PPIs. | |
| Prevention measures: | Temporary discontinuation of Dexilant™ may be considered in some patients receiving high doses of methotrexate. | |
| Digoxin | ||
| Clinical impact: | Potential for increased digoxin exposure. | |
| Prevention measures: | Monitor digoxin concentrations. Digoxin dosage adjustments may be required to maintain therapeutic drug concentrations. See instructions for use of digoxin. | |
| Drugs whose absorption is dependent on pH (eg, iron salts, erlotinib, dasatinib, nilotinib, mycophenolate mofetil, ketoconazole/itraconazole) | ||
| Clinical impact: | Dexlansoprazole may reduce the absorption of other drugs due to its effect on reducing stomach acid. | |
| Prevention measures: | Mycophenolate mofetil (MMF): When MMF was coadministered with a PPI in healthy individuals and organ transplant patients, decreased exposure to the active metabolite and mycophenolic acid (MPA) was observed, possibly due to decreased solubility of MMF at elevated gastric pH. In transplant patients receiving Dexilant™ and MMF, the clinical significance of reducing the effects of MPA on organ rejection has not been established. Transplant patients receiving MMF should use Dexilant™ with caution. See instructions for use of other medications whose absorption is dependent on gastric pH. | |
| Tacrolimus | ||
| Clinical impact: | Potentially higher exposure to tacrolimus, particularly in transplant patients who are intermediate or poor metabolizers of CYP2C19. | |
| Prevention measures: | Monitor tacrolimus blood concentrations. Dose adjustments of tacrolimus may be necessary to maintain therapeutic drug concentrations. See instructions for use of tacrolimus. | |
| Interaction with diagnostic tests for neuroendocrine tumors | ||
| Clinical impact: | Secondary increase in CgA levels due to PPI-induced decrease in gastric acidity. Elevated CgA levels may cause false-positive results in diagnostic tests for neuroendocrine tumors. | |
| Prevention measures: | Temporarily stop taking Dexilant™ at least 14 days before assessing CgA levels and repeat the test if baseline CgA levels are high. If a series of tests are performed (for example, for monitoring), they should be performed in the same laboratory, since reference ranges may differ between tests. | |
| Interaction with secretin stimulation test | ||
| Clinical impact: | A hyperresponse of gastrin secretion to a secretin stimulation test may falsely suggest gastrinoma. | |
| Prevention measures: | Temporarily discontinue treatment with Dexilant™ at least 30 days before evaluation to allow gastrin levels to return to baseline. | |
| False-positive urine test results for tetrahydrocannabinol | ||
| Clinical impact: | False-positive tetrahydrocannabinol (THC) urine test results have been observed in patients receiving PPIs. | |
| Prevention measures: | To confirm positive results, an alternative test should be considered. | |
| CYP 2 C drugs and CYP 3 A 4 | |
| Clinical impact: | Reduced exposure to dexlansoprazole when used concomitantly with strong inducers. |
| Prevention measures: | St. John's wort, rifampin: Avoid use with Dexilant™. Medicines containing ritonavir: see instructions for use. |
| Inhibitors of CYP2C19 or CYP3A4 | |
| Clinical impact: | Increased exposure of dexlansoprazole is expected when used concomitantly with strong inhibitors. |
| Prevention measures: | Voriconazole: See instructions for use. |
Effect of dexlansoprazole on other drugs
Cytochrome P 450
Dexlansoprazole is partially metabolized by CYP2C19 and CYP3A4.
In vitro studies have shown that dexlansoprazole does not inhibit CYP isoforms 1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2D6, 2E1 or 3A4. Therefore, any clinically significant interactions with drugs that are metabolized by these CYP enzymes are not expected. In addition, in vivo studies have shown that Dexilant™ does not affect the pharmacokinetics of phenytoin (CYP2C9 substrate) or theophylline (CYP1A2 substrate). Although in vitro studies have shown that Dexilant™ may inhibit CYP2C19 in vivo; An in vivo drug interaction study among extensive and intermediate metabolizers of CYP2C19 showed that Dexilant™ does not affect the pharmacokinetics of diazepam (a CYP2C19 substrate).
Clopidogrel
Clopidogrel is partially metabolized to its active metabolite by CYP2C19. A study was conducted in healthy subjects who were extensive metabolizers of CYP2C19 and took clopidogrel 75 mg alone or concomitantly with Dexilant™ 60 mg (n = 40), once daily for nine days. The mean AUC of the active metabolite of clopidogrel was reduced by approximately 9% when clopidogrel was administered with Dexilant™. Concomitant administration of dexlansoprazole and clopidogrel in healthy subjects did not have a clinically significant effect on exposure to the active metabolite of clopidogrel or clopidogrel-induced platelet inhibition.
Effect of other drugs on dexlansoprazole
Because dexlansoprazole is metabolized by CYP2C19 and CYP3A4, inducers and inhibitors of these enzymes may potentially alter the exposure of dexlansoprazole.
special instructions
Gastric neoplasms
A symptomatic response to Dexilant™ does not exclude the presence of gastric malignancy.
Acute interstitial nephritis
Acute interstitial nephritis has been observed in patients taking PPIs, including lansoprazole. Acute interstitial nephritis can occur at any time during PPI therapy, usually associated with an idiopathic hypersensitivity reaction. If acute interstitial nephritis develops, treatment with Dexilant™ should be discontinued.
Cyanocobalamin (vitamin B12) deficiency
Daily treatment with any acid-suppressing drugs for a long period of time (for example, more than 3 years) can lead to impaired absorption of cyanocobalamin (vitamin B12), caused by hypo- or achlorhydria. Rare reports of cyanocobalamin deficiency due to acid suppression therapy have been described. This diagnosis should be considered in patients taking Dexilant™ if clinical symptoms suggestive of cyanocobalamin deficiency are observed.
Clostridial diarrhea
Published observational studies suggest that PPI therapy, such as Dexilant™ therapy, may be associated with an increased risk of clostridial diarrhea, particularly in hospitalized patients. This diagnosis should be considered for diarrhea that does not improve.
Patients should use the lowest dose and shortest duration of PPI therapy appropriate for the condition being treated.
Bone fracture
A number of published observational studies suggest that PPI therapy may be associated with an increased risk of hip, wrist, or spine fractures associated with osteoporosis. The risk of fracture is increased in patients receiving high doses, determined by