Home | About us | Delivery | Advertisers | Login | Registration
Delivery on Sundays and holidays does not work!
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2022 Pharmacy 84.
Meloxicam-Teva
Patients suffering from diseases of the gastrointestinal tract should be monitored regularly. If ulcerative lesions of the gastrointestinal tract or gastrointestinal bleeding occur, Meloxicam-Teva should be discontinued.
Gastrointestinal ulcers, perforation, or bleeding may occur at any time during the use of NSAIDs, with or without warning symptoms or a history of serious gastrointestinal complications. The consequences of these complications are generally more serious in older people.
When using the drug Meloxicam-Teva, serious skin reactions may develop, such as exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis. In this regard, special attention should be paid to patients who report the development of adverse events from the skin and mucous membranes, as well as hypersensitivity reactions to the drug, especially if such reactions were observed during previous courses of treatment. The development of such reactions is observed, as a rule, during the first month of treatment. If the first signs of a skin rash, changes in the mucous membranes or other signs of hypersensitivity appear, discontinuation of the use of Meloxicam-Teva should be considered.
Cases have been described when taking NSAIDs to increase the risk of developing serious cardiovascular thrombosis, myocardial infarction, angina, possibly fatal. This risk increases with long-term use of the drug, as well as in patients with a history of the above diseases and predisposed to such diseases.
NSAIDs inhibit the synthesis of prostaglandins in the kidneys, which are involved in maintaining renal perfusion. The use of NSAIDs in patients with reduced renal blood flow or reduced circulating blood volume may lead to decompensation of latent renal failure. After discontinuation of NSAIDs, renal function usually returns to baseline levels. Elderly patients are most at risk of developing this reaction; patients with dehydration, chronic heart failure, liver cirrhosis, nephrotic syndrome or acute renal dysfunction; patients simultaneously taking diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists; as well as patients who have undergone major surgical procedures that lead to hypovolemia. In such patients, diuresis and renal function should be carefully monitored when initiating therapy.
The use of NSAIDs in combination with diuretics can lead to sodium, potassium and water retention, as well as a decrease in the natriuretic effect of diuretics. As a result, predisposed patients may experience increased signs of heart failure or hypertension. Therefore, such patients must be closely monitored and adequate hydration maintained. Before starting treatment, a kidney function test is necessary.
In case of combination therapy, renal function should also be monitored.
When using the drug Meloxicam-Teva (as well as most other NSAIDs), an episodic increase in the activity of transaminases in the blood serum or other indicators of liver function is possible. In most cases, this increase was small and transitory. If the identified changes are significant or do not decrease over time, the drug Meloxicam-Teva should be discontinued and the identified laboratory changes should be monitored.
Weakened or malnourished patients may be less able to tolerate adverse events and should be monitored closely.
Like other NSAIDs, Meloxicam-Teva can mask the symptoms of an underlying infectious disease.
As a drug that inhibits cyclooxygenase/prostaglandin synthesis, Meloxicam-Teva may have an effect on fertility and is therefore not recommended for women who have difficulty conceiving. In this regard, in women undergoing examination for this reason, it is recommended to discontinue the drug Meloxicam-Teva.
In patients with mild or moderate renal impairment (creatinine clearance more than 25 ml/min), no dose adjustment is required.
In patients with liver cirrhosis (compensated), no dose adjustment is required.
Side effects
Frequency of side effects: often (more than 1%); uncommon (0.1-1%); rarely (0.01-0.1%); very rare (less than 0.01%).
From the digestive system:
often - dyspepsia, incl. nausea, vomiting, abdominal pain, constipation, flatulence, diarrhea; infrequently - transient increase in the activity of liver transaminases, hyperbilirubinemia, belching, esophagitis, gastroduodenal ulcer, bleeding from the gastrointestinal tract (including hidden), stomatitis; rarely - gastrointestinal perforation, colitis, hepatitis, gastritis.
From the hematopoietic organs:
often - anemia; infrequently - changes in the blood formula, incl. leukopenia, thromocytopenia.
From the skin:
often - itching, skin rash; infrequently - urticaria; rarely - photosensitivity, bullous rashes, erythema multiforme, incl. Stevens-Johnson syndrome, toxic epidermal necrolysis.
From the respiratory system:
infrequently - the occurrence of attacks of bronchial asthma in persons allergic to acetylsalicylic acid or other NSAIDs.
From the side of the central nervous system:
often - dizziness, headache; uncommon - vertigo, tinnitus, drowsiness; rarely - confusion, disorientation, emotional lability.
From the cardiovascular system:
often - peripheral edema; infrequently - increased blood pressure, palpitations, flushes of blood to the skin of the face.
From the urinary system:
uncommon - hypercreatininemia and/or increased urea concentration in the blood serum; rarely - acute renal failure; The connection with taking meloxicam has not been established - interstitial nephritis, albuminuria, hematuria.
From the senses:
rarely - conjunctivitis, visual impairment, incl. blurred visual perception.
Allergic reactions:
rarely - angioedema, anaphylactoid/anaphylactic reactions.
Drug interactions
- Pharmacodynamic interaction:
The simultaneous use of several NSAIDs (including salicylates) may increase the risk of developing erosive and ulcerative lesions of the gastrointestinal tract due to a synergistic effect, therefore the use of meloxicam with other NSAIDs is not recommended.
When using meloxicam with diuretics, the patient must drink sufficient fluids, and regular medical monitoring of renal function is required before and during treatment.
Combined use with indirect anticoagulants increases the risk of bleeding due to inhibition of platelet function and damage to the mucous membrane of the stomach and intestines. Therefore, combined use with NSAIDs and indirect anticoagulants is not recommended.
With the combined use of thrombolytic and antithrombotic drugs with meloxicam, an increased risk of bleeding is possible (periodic monitoring of blood coagulation parameters is necessary).
Concomitant use with ACE inhibitors and other antihypertensive drugs in elderly patients with symptoms of dehydration can provoke the development of acute renal failure. In addition, combined use with meloxicam may reduce their hypotensive effect.
Meloxicam enhances the nephrotoxic effect of cyclosporine.
Meloxicam may reduce the effectiveness of contraceptives.
Pharmacokinetic interaction
NSAIDs may increase serum lithium concentrations to toxic levels (decreased renal excretion of lithium). Therefore, the simultaneous use of meloxicam with lithium preparations is not recommended. If their combined use is necessary, the lithium content in the blood serum should be carefully monitored before, during and after the end of the course of therapy with meloxicam and lithium preparations.
When used simultaneously with methotrexate, the negative effect on the hematopoietic system increases (the risk of developing anemia and leukopenia). Periodic monitoring of the hemogram is necessary.
Cholestyramine accelerates the elimination of meloxicam, increasing the clearance of meloxicam by 50%, reducing its T1/2 by 13±3 hours. This interaction is of clinical significance.
When taken simultaneously with antacids, cimetidine and digoxin, no significant clinical interaction is observed.
NSAIDs reduce the effectiveness of contraceptive intrauterine devices.
When using meloxicam together with drugs that have a known ability to inhibit CYP2C9 and/or CYP3A4 (or are metabolized by these enzymes), the possibility of pharmacokinetic interaction should be taken into account. The possibility of interaction of meloxicam with oral hypoglycemic agents cannot be excluded.
When meloxicam is used simultaneously with selective serotonin reuptake inhibitors, the risk of gastrointestinal bleeding increases.
Overdose
Increased dose-dependent side effects.
Symptoms:
impaired consciousness, nausea, vomiting, epigastric pain, bleeding from the gastrointestinal tract, acute renal failure, acute liver failure, respiratory arrest, asystole.
Treatment:
symptomatic therapy. Forced diuresis, alkalinization of urine, hemodialysis are ineffective due to the high degree of binding of meloxicam to plasma proteins. There is no specific antidote
Directions for use and doses
The drug is administered deep intramuscularly. IV is contraindicated.
IM administration of Meloflex Rompharm is indicated only during the first days of treatment. Subsequently, treatment is continued with oral forms (tablets).
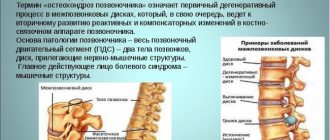
Osteoarthritis in the acute phase - 7.5 mg/day; if the condition does not improve, the dose can be increased to 15 mg/day.
Rheumatoid arthritis, ankylosing spondylitis - 15 mg/day. Depending on the therapeutic effect, the dose can be reduced to 7.5 mg/day. The daily dose of meloxicam should not exceed 15 mg.
In elderly patients, the recommended dose for long-term therapy of rheumatoid arthritis or ankylosing spondylitis is 7.5 mg/day. In elderly patients with an increased risk of side effects, therapy should be started with a dose of 7.5 mg/day.
In dialysis patients with severe renal impairment, the daily dose of meloxicam should not exceed 7.5 mg. In patients with mild or moderate renal impairment (creatinine clearance more than 25 ml/min), there is no need to reduce the dose.
In patients with mild or moderately severe liver dysfunction, there is no need to reduce the dose of Meloflex Rompharm.