Write a review
Reviews: 0
Manufacturers: Pliva-Lachema (Czech Republic)
Active ingredients
- Paclitaxel
Disease class
- Malignant neoplasm of the esophagus
- Malignant neoplasm of the stomach
- Malignant neoplasm of the bronchi and lungs
- Kaposi's sarcoma
- Malignant neoplasm of the breast
- Malignant neoplasm of the cervix
- Cancer of the triangle of the bladder
- Cancer of the head, face, neck
- Leukemia of unspecified cell type
Clinical and pharmacological group
- Not indicated. See instructions
Pharmacological action
- Antitumor
Pharmacological group
- Antitumor agents of plant origin
Concentrate for the preparation of solution for intravenous administration of Paclitaxel (Paclitaxel)
Instructions for medical use of the drug
Description of pharmacological action
It has a cytotoxic antimitotic effect. Activates the assembly of microtubules from tubulin dimers and stabilizes them, protecting them from depolymerization. As a result, it inhibits the dynamic reorganization of the microtubular network in interphase and during mitosis. Induces an abnormal arrangement of microtubules in the form of bundles throughout the cell cycle and multiple star-shaped clusters (asters) during mitosis.
Indications for use
Ovarian cancer, breast cancer, non-small cell lung cancer, squamous cell cancer of the head and neck, transitional cell bladder cancer, esophageal cancer, leukemia, Kaposi's sarcoma in patients with AIDS.
Release form
Concentrate for the preparation of solution for infusion 6 mg/ml; bottle (bottle) 16.7 ml cardboard pack 1; Concentrate for the preparation of solution for infusion 6 mg/ml; bottle (bottle) 5 ml cardboard pack 1; Concentrate for the preparation of solution for infusion 6 mg/ml; bottle (bottle) 5 ml cardboard pack 10;
Pharmacodynamics
It has a cytotoxic antimitotic effect. Activates the assembly of microtubules from tubulin dimers and stabilizes them, protecting them from depolymerization. As a result, it inhibits the dynamic reorganization of the microtubular network in interphase and during mitosis. Induces an abnormal arrangement of microtubules in the form of bundles throughout the cell cycle and multiple star-shaped clusters (asters) during mitosis.
Pharmacokinetics
The pharmacokinetic parameters of paclitaxel were determined after infusions of the drug at doses of 135 and 175 mg/m2 over 3 and 24 hours in randomized Phase 3 studies in patients with ovarian cancer. When administered intravenously for 3 hours at a dose of 135 mg/m2, Cmax was 2170 ng/ml, AUC was 7952 ng/h/ml; when the same dose is administered over 24 hours, it is 195 ng/ml and 6300 ng/h/ml, respectively. Cmax and AUC are dose-dependent. With a 3-hour infusion, increasing the dose by 30% (from 135 to 175 mg/m2) leads to an increase in Cmax and AUC by 68 and 89%, respectively; with a 24-hour infusion, Cmax increases by 87% and AUC by 26%. In vitro studies have shown that at paclitaxel concentrations of 0.1–50 μg/ml, 89–98% of the substance is bound to serum proteins. After intravenous administration of paclitaxel, the dynamics of the decrease in plasma concentration is biphasic: the initial rapid decrease reflects distribution in the tissue and its significant elimination. The later phase is due in part to the relatively slow release of paclitaxel from tissue. With intravenous administration, the half-life of distribution from the blood into tissues is on average 30 minutes. The apparent volume of distribution at steady state during a 24-hour infusion is 227–688 l/m2. Easily penetrates and is adsorbed by tissues, mainly accumulates in the liver, spleen, pancreas, stomach, intestines, heart, and muscles. The metabolism of paclitaxel in humans has not been fully studied. After IV infusion (1–24 hours), the average cumulative urinary excretion of unchanged drug is 1.3–12.6% of the dose (15–275 mg/m2), indicating extensive extrarenal clearance. It has been shown that paclitaxel is metabolized in animals by the liver. Probably the main mechanism of metabolism of paclitaxel in the human body is biotransformation in the liver and excretion in the bile. The main metabolites are hydroxylation products. The effect of renal or hepatic impairment on metabolism following a 3-hour infusion has not been studied. Pharmacokinetic parameters obtained in one patient indicate that dialysis does not affect the rate of drug elimination from the body. T1/2 and total clearance are variable (depending on the dose and duration of intravenous administration): at doses of 135–175 mg/m2 and an infusion duration of 3 or 24 hours, the average T1/2 values are in the range of 13.1–52.7 hours , ground clearance - 12.2–23.8 l/h/m2. Does not accumulate with repeated infusions.
Use during pregnancy
Contraindicated during pregnancy (possible embryo- and fetotoxic effects). FDA category of effect on the fetus is D. Breastfeeding should be discontinued during treatment (it is unknown whether paclitaxel passes into breast milk).
Contraindications for use
Hypersensitivity (including to polyoxyethylene castor oil), severe neutropenia - less than 1.5 109/l (initial or developed during treatment), in patients with Kaposi's sarcoma - neutropenia less than 1.0 109/l (initial or developed during treatment).
Side effects
Based on pooled data from 10 studies involving 812 patients (493 ovarian cancer, 319 breast cancer), the following side effects were observed using different doses and durations of paclitaxel administration. Hematological: neutropenia less than 2·109/l (90%), neutropenia less than 0.5·109/l (52%), leukopenia less than 4·109/l (90%), leukopenia less than 1·109/l (17% ), thrombocytopenia less than 100·109/l (20%), thrombocytopenia less than 50·109/l (7%), anemia - hemoglobin level less than 110 g/l (78%), anemia - hemoglobin level less than 80 g/l ( 16%). Bone marrow suppression (mainly neutropenia) is the main dose-limiting toxic effect of paclitaxel. Neutropenia depends less on the dose of the drug and more on the duration of administration (more pronounced with a 24-hour infusion). The lowest level of neutrophils is usually observed on days 8–11 of treatment, normalization occurs on day 22. An increase in temperature was observed in 12% of patients, infectious complications - in 30% of patients. Death was recorded in 1% of patients diagnosed with sepsis, pneumonia and peritonitis. The most common infections associated with neutropenia are urinary and upper respiratory tract infections. When thrombocytopenia develops, the lowest platelet level is usually observed on days 8–9 of treatment. Bleeding (14% of cases) was local, the frequency of its occurrence was not related to the dose and time of administration. The frequency and severity of anemia did not depend on the dose and mode of administration of paclitaxel. Red blood cell transfusion was required in 25% of patients, and platelet transfusion in 2% of patients. In patients with Kaposi's sarcoma that developed against the background of AIDS, suppression of bone marrow hematopoiesis, infections, and febrile neutropenia may occur more frequently and have a more severe course. Hypersensitivity reactions. The frequency and severity of hypersensitivity reactions did not depend on the dose or mode of administration of paclitaxel. All patients during clinical studies received adequate premedication before administration of paclitaxel. Hypersensitivity reactions were observed in 41% of patients and mainly manifested themselves in the form of flushing (28%), rash (12%), arterial hypotension (4%), shortness of breath (2%), tachycardia (2%) and arterial hypertension (1%). Severe hypersensitivity reactions that required therapeutic intervention (shortness of breath requiring the use of bronchodilators, arterial hypotension requiring therapeutic intervention, angioedema, generalized urticaria) were observed in 2% of cases. These reactions are likely histamine-mediated. In case of severe hypersensitivity reactions, the drug infusion should be stopped immediately and symptomatic treatment should be started, and the drug should not be re-administered. Cardiovascular. Hypotension (12%, n=532) or hypertension and bradycardia (3%, n=537) were noted during drug administration. Severe side effects were observed in 1% of cases, which included syncope, cardiac arrhythmias (asymptomatic ventricular tachycardia, bigeminy and complete AV block and syncope), hypertension and venous thrombosis. One patient with syncope developed progressive hypotension with fatal outcome during a 24-hour infusion of paclitaxel 175 mg/m2. ECG abnormalities were also observed in clinical trials (23%). In most cases, there was no clear association between the use of paclitaxel and ECG changes, the changes were not clinically significant or had minimal clinical significance. In 14% of patients with normal ECG parameters before inclusion in the study, ECG abnormalities that arose during treatment were noted. Neurological. The frequency and severity of neurological manifestations were dose-dependent, but were not influenced by the duration of infusion. Peripheral neuropathy, mainly manifested in the form of paresthesia, was observed in 60% of patients, in severe form - in 3% of patients, in 1% of cases it was the reason for discontinuation of the drug. The incidence of peripheral neuropathy increased with increasing total dose of paclitaxel. Symptoms usually appear after repeated use and subside or resolve within a few months of stopping treatment. Pre-existing neuropathy due to previous treatment is not a contraindication to paclitaxel therapy. Other serious neurological disorders observed after administration of paclitaxel (less than 1% of cases): grand mal seizures, ataxia, encephalopathy. There are reports of neuropathy at the level of the autonomic nervous system, which has led to paralytic ileus. Arthralgia/myalgia was observed in 60% of patients and was severe in 8% of patients. Symptoms were usually transient, appearing 2–3 days after paclitaxel administration and resolving within a few days. Hepatotoxicity. Increased serum levels of AST, alkaline phosphatase and bilirubin were observed in 19% (n=591), 22% (n=575) and 7% (n=765) of patients, respectively. Cases of liver necrosis and encephalopathy of hepatic origin with a fatal outcome have been described. Gastrointestinal. Nausea/vomiting, diarrhea and mucositis were observed in 52, 38 and 31% of patients, respectively, and were mild or moderate in nature. Mucositis was observed more frequently with the 24-hour infusion than with the 3-hour infusion. In addition, phenomena of intestinal obstruction or perforation, neutropenic enterocolitis (typhlitis), and thrombosis of the mesenteric artery (including ischemic colitis) were observed. Reactions at the IV injection site (13%): local swelling, pain, erythema, induration. These reactions are more likely to occur after a 24-hour infusion than after a 3-hour infusion. Currently, there are no specific forms of treatment for reactions associated with drug extravasation. There are reports of the development of phlebitis and cellulite with the administration of paclitaxel. Other toxic manifestations. Reversible alopecia was observed in 87% of patients. Complete hair loss is observed in almost all patients between 14–21 days of therapy. Pigmentation disturbances or discoloration of the nail bed occurred (2%). Transient skin changes due to increased sensitivity to paclitaxel have also been observed. Edema was reported in 21% of patients, incl. in 1% - in a pronounced form, but these cases were not the reason for discontinuation of the drug. In most cases, the edema was focal and related to the disease. There have been reports of recurrence of radiation-related skin reactions.
Directions for use and doses
IV, in the form of a 3-hour or 24-hour infusion (immediately before administration, the drug is diluted to a concentration of 0.3–1.2 mg/ml with appropriate solutions). The dosage regimen is set individually, depending on the indications, previous chemotherapy (or lack thereof), the state of the hematopoietic system, and the chemotherapy regimen.
Overdose
Symptoms: myelosuppression, peripheral neurotoxicity, mucositis. Treatment: symptomatic. A specific antidote is unknown.
Interactions with other drugs
According to clinical studies, when paclitaxel was administered after cisplatin infusion, more pronounced myelosuppression and a decrease in paclitaxel clearance by approximately 33% were observed compared with the reverse sequence of administration (paclitaxel before cisplatin). In in vitro studies, ketoconazole inhibits the biotransformation of paclitaxel. Cimetidine, ranitidine, dexamethasone, diphenhydramine do not affect the binding of paclitaxel to plasma proteins. Inhibitors of microsomal oxidation (including ketoconazole, cimetidine, quinidine, cyclosporine) inhibit the metabolism of paclitaxel.
Precautions for use
Treatment should be carried out by a doctor experienced in chemotherapy, and in the presence of conditions necessary to relieve complications. Constant monitoring of peripheral blood, blood pressure, heart rate and other parameters of vital functions is required (especially during the initial infusion or during the first hour of administration). When using paclitaxel in combination with cisplatin, paclitaxel should be administered first followed by cisplatin. To avoid severe hypersensitivity reactions (and to improve tolerability), all patients should be premedicated with glucocorticoids, antihistamines, and histamine H2 receptor blockers prior to infusion; approximate regimen: dexamethasone (or analogue) - 20 mg orally or intramuscularly 6-12 hours before administration of paclitaxel, diphenhydramine (or analogue) - 50 mg intravenously and cimetidine - 300 mg (or ranitidine - 50 mg) intravenously c 30–60 minutes before administration. If severe allergic reactions occur during the infusion, the administration is immediately stopped and symptomatic therapy is carried out. If neutropenia develops, patients should not be re-administered the drug until the neutrophil content is restored to a level of at least 1.5 109/L and at least 1 109/L for Kaposi's sarcoma (see “Contraindications”). If severe neuropathic peripheral disorders or severe neutropenia (less than 0.5·109/l) develop as a result of paclitaxel infusion, incl. lasting 7 days or more or accompanied by infectious complications, if repeated courses are necessary, it is recommended to reduce the dose by 20%. If significant cardiac conduction disturbances occur during treatment with paclitaxel, appropriate treatment should be prescribed, and upon subsequent administration of the drug, continuous monitoring of cardiac function should be performed. During the treatment period, it is recommended to refrain from engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions. During treatment, it is recommended to use adequate methods to prevent pregnancy.
Special instructions for use
When working with paclitaxel, as when working with other anticancer drugs, caution must be exercised. The preparation of solutions should be carried out by trained personnel in a specially designated area, observing protective measures (gloves, masks, etc.). In case of contact of the drug with the skin or mucous membranes, it is necessary to thoroughly wash the mucous membranes with water, and the skin with soap and water. When preparing, storing and administering paclitaxel, use equipment that does not contain PVC parts.
Storage conditions
List B: In a place protected from light, at a temperature not exceeding 25 °C.
Best before date
24 months
ATX classification:
L Antineoplastic drugs and immunomodulators
L01 Antineoplastic drugs
L01C Alkaloids of plant origin
L01CD Taxoids
L01CD01 Paclitaxel
Paclitaxel and its new possibilities in the treatment of patients with ovarian cancer
IN
The high frequency of disseminated forms of ovarian cancer (OC) with a steady increase in incidence determines the relevance of the problem of drug treatment of this pathology.
The high mortality rate of patients with ovarian cancer is due not only to the rarity of detection of the disease in the early stages, but also to the insufficient use of the most effective combined and complex treatment methods. According to the latest statistics, in Russia only 70% of patients with ovarian cancer receive the necessary combined or complex treatment
, including not only surgical treatment, but also modern antitumor drug and radiation therapy. The role of chemotherapy in the treatment of ovarian cancer has already been well studied and has its own standards, which is largely explained by the high chemosensitivity of this tumor. Increasing the effectiveness of treatment of patients with ovarian cancer is associated with the search for new antitumor drugs. Since the mid-90s, these have been drugs of a new generation - taxanes.
Over 10 years of using paclitaxel in the field of clinical chemotherapy of tumor diseases, the main indications and regimens have already been developed for the treatment of patients with cancer of the breast, lung, ovaries, oral mucosa, oropharynx, nasopharynx, and larynx. Such a wide range of antitumor activity makes it possible to look for new possibilities for the use of paclitaxel in platinum-resistant forms of ovarian cancer, as well as to study its effectiveness in new localizations and rare histological variants of malignant tumors. As a result of the creation of new and improvement of “old” chemotherapy regimens, there is a gradual increase in the effectiveness of treatment and improvement in long-term results. Thus, Figure 1 presents data from a retrospective analysis of the treatment of disseminated patients with ovarian cancer at the State Institution Russian Cancer Research Center named after. N.N. Blokhin RAMS from 1979 to 2000. When analyzing the effectiveness of combination treatment depending on the chemotherapy regimens used, we can trace the evolution of treatment of primary patients with advanced ovarian cancer over the past 20 years. However, we must emphasize that the interpretation of the results of the chemotherapy performed is somewhat difficult, since the patients were treated at different times and received a variety of regimens. Thus, when using platinum-free combinations, treatment was effective in 54%, and the number of complete regressions was 25%. In the 80s, platinum drugs appeared, which retain a strong position in combination chemotherapy for ovarian cancer to this day. Their use made it possible to achieve an overall efficiency of 64%, and the number of complete regressions was already 35%. In the 90s, fundamentally new drugs for the drug therapy of OC, such as paclitaxel and docetaxel, were introduced into world clinical practice. The introduction of taxanes increased the effectiveness of treatment to 79%, and the number of complete regressions increased to 46%.
Rice. 1. The effectiveness of combination treatment of patients with ovarian cancer
The purpose of this article is to summarize the accumulated experience in the use of paclitaxel, as well as to determine the prospects for research programs in combination chemotherapy for ovarian cancer.
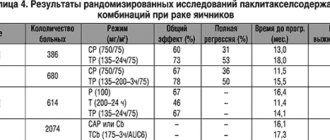
Paclitaxel is the first drug from the taxane group to demonstrate high activity (16–50%) in the treatment of platinum-resistant ovarian cancers [1,2]. Its widespread use in the United States in patients with ovarian cancer began in December 1992, and already in April 1998, in combination with carboplatin, it was approved by the FDA (Specialized Administrative Commission on the Approval of New Drugs for Use in Clinical Practice) for first-line chemotherapy of ovarian cancer and firmly established in the standards of treatment. All this time, the study of paclitaxel in various combinations and dosages continued, and optimal treatment regimens for patients with ovarian cancer were developed. The results of the largest studies are presented in Table 1.
Based on data from randomized trials, paclitaxel with cisplatin or carboplatin has been actively introduced into daily clinical practice. However, data from the ICON-3 protocol, conducted in 1995–98, showed that the SAR regimen and carboplatin alone as 1st line are not inferior in long-term results to paclitaxel with carboplatin, and when taking into account side effects, it is even preferable for patients with ovarian cancer with I –IIIa stages. The advantage of regimens with paclitaxel appeared only for patients with an unfavorable prognosis, that is, with residual tumor more than 1 cm [11].
The results of the protocols conducted do not answer many questions that concern clinicians, so new studies are being conducted with specific narrow goals, for example: GOG 157
and
GOG 175
should show the role of paclitaxel in the early stages of OC (stages I–II) in patients with a high risk of developing a relapse of the disease;
SWOG
,
GOG 178
protocols should demonstrate the role of consolidation therapy with paclitaxel in standard and weekly regimens in achieving complete remission in patients with disseminated ovarian cancer;
EORTC-GCG
study will demonstrate the effectiveness of a new regimen of paclitaxel with topotecan, and will also determine the role and location of cytoreductive surgery in treatment with paclitaxel and cisplatin.
In addition to expanding knowledge during phase III clinical trials, the experiment revealed therapeutic synergy between paclitaxel and gemcitabine, topotecan, fluorouracil, cisplatin, cyclophosphamide, etoposide, and vincristine [12]. This circumstance and the high activity of paclitaxel in monochemotherapy in patients with ovarian cancer served as the basis for studying new treatment regimens. The study data presented in Table 2 were not randomized. Rather, this is an attempt to optimize the treatment of patients with ovarian cancer with unfavorable prognosis factors.
The results of using paclitaxel in combination with cisplatin or carboplatin, as well as with other drugs in untreated patients, are superior to the effectiveness of the standard combination of cisplatin and cyclophosphamide. More aggressive paclitaxel regimens were studied compared to paclitaxel 175 mg/m2 and carboplatin AUC6. Thus, two courses of carboplatin AUC9, and then 6 courses of paclitaxel 135 mg/m2/24-hour IV infusion and cisplatin 100 mg/m2 intraperitoneally demonstrated an advantage only in the duration of the effect. A German-Franco-Austrian study compares the combinations of paclitaxel 175 mg/m2 with carboplatin AUC6 - 117 patients and carboplatin AUC6 + paclitaxel 175 mg/m2 + epirubicin (before paclitaxel) 60 mg/m2 - 111 patients. When assessing the effectiveness of treatment, no significant differences were obtained (PR-42 and 48%, PR-30 and 38%, respectively); toxicity was higher when treated with a three-component regimen [21].
The results of treatment of 35 patients with advanced ovarian cancer with paclitaxel on day 1 at a dose of 200 mg/m2/3-hour infusion, cisplatin on day 2 70 mg/m2 and on days 1, 2, 3 with ifosfamide 1.5 g/ m2. The effectiveness of this combination was 86% (CR–59%), the median time to progression was 23 months [19].
In our opinion, regimens combining paclitaxel with gemcitabine are very interesting and promising. Thus, the regimen: paclitaxel 175 mg/m2 on day 1, carboplatin AUC 5 on day 1 and gemcitabine 800 mg/m2 on days 1 and 8, according to preliminary data, is effective in 100% of primary patients with III-IV ovarian cancer stages of the disease [14]. This combination has also been successfully studied in the treatment of patients with recurrent ovarian cancer [20]. A new combination of topotecan 0.75 - 1 mg/m2 on days 1–3, paclitaxel 175 mg/m2/3-hour infusion on day 3, and carboplatin AUC 5 on day 3 demonstrated 88.2% effectiveness. (CR–23.5%) patients (17 patients were assessed). The main limiting toxicity of this regimen is hematological [17,18].
Taking into account the high effectiveness of paclitaxel in ovarian cancer, it should be included in first-line chemotherapy regimens, especially if the patient has several unfavorable prognostic factors.
In case of progression or stabilization of the disease on standard treatment (SR) or in case of early relapse of the disease, 2nd line chemotherapy regimens must necessarily include paclitaxel
.
At the Russian Scientific Research Center named after. N.N. Blokhin Russian Academy of Medical Sciences continues to study a 2nd-line regimen of drug therapy for OC: paclitaxel (abitaxel) 120–135 mg/m2/3-hour infusion on day 1, carboplatin AUC 5 on day 1 and altretamine per os
160 mg/m2 /day 2–15 days. The limiting toxicity of this regimen is hematological (thrombocytopenia, leukopenia, anemia), therefore, for the first course of treatment, the patient receives paclitaxel 120 mg/m2, and if well tolerated, the dosage can be increased to 135 mg/m2. Preliminary results of using this combination showed its promise and good tolerability, and the objective clinical effect was 70.4%. The combination is well tolerated by elderly patients.
Thus, all cooperative studies demonstrated the high effectiveness of paclitaxel–platinum-containing regimens
, as well as three-component regimens when conducting 2 lines of treatment. The use of these combinations may be limited by the occurrence of myelotoxicity and neurotoxicity.
At the State Scientific Research Center named after. N.N. Blokhin RAMS, GUN Research Institute of Oncology named after. prof. N.N. Petrov Ministry of Health of the Russian Federation and the Sverdlovsk Regional Oncology Center of the Oncology Scientific and Practical Center are conducting a clinical trial of paclitaxel, manufactured by Dr. Reddy's Laboratories Ltd. Mitotax
®
, produced by the pharmaceutical company Dr. Reddy's. It is structurally identical to paclitaxel, an antitumor drug, and has similar activity. The drug is available in convenient packaging: in bottles of 30 mg/5 ml, 100 mg/16.7 ml and 250 mg/41.7 ml. Composition: active substance: paclitaxel, 1 ml of concentrate contains 6 mg of active substance. Excipients: polyoxyl 35, castor oil, absolute alcohol. In a preliminary analysis of the experience of using Mitotax in patients with ovarian cancer, the spectrum of antitumor activity and toxicity is completely similar to paclitaxel, which allows us to recommend the drug for widespread use in clinical practice [21]. References:
1. Ten Bokkel Huinink W, Gore W., et al., J. Clin. Oncol. 1997; 15:2183–2193.
2. Canetta R. The development of new cytotoxic drugs for ovarian cancer: review of literature and methodological aspects. Forum 1994:4:702–720.
3. Stuart G., Bertelsen K., Mangioni C., et al. Updated analysis shows a highly significant improved overall survival (OS) for cisplatin–paclitaxel as first line treatment of advanced OC: mature results of the EORTC–GCCG, NOCOVA, NC 1C CTG and Scottish Intergroup Trial. Proc. Am. Soc. Clin. Oncol (ASCO) 1998: 17:361a (abs.1394)
4. McGuire WP, Hoskins WJ, Brady MP et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and IV ovarian cancer. N.Engl. Med. 1996,334:1–6.
5. Muggia F., Brady M., Sutton G., et. al. Phase III trial of cisplatin or paclitaxel vr their combination in suboptimal stage III and IV epithelial OC. Gynecologic Oncology Group study 132., Proc. ASCO, 1997 16:352a.
6. Ozols RF, Bundy B, Fowler J, et al. Randomized phase III study of cisplatin vs carboplatin in optimal stage III OC: a GOG study. Proc. ASCO, 1999, 18: 356a
7. Ozols RF Ovarian Cancer (Current Status and Future Directions). In: Progress in Anti-cancer Chemotherapy. Ed. By D. Khayat and C. N. Hortobagye. Springer-Verlag. France, 2000, pp. 135–144.
8. Neijt JP, Hansen M, et al. Randomized phase III study in previously untreated epithelial OC FIGO stage IIb, IIc, III, IV comparing paclitaxel–cisplatin and paclitaxel–carboplatin. Proc. ASCO 1997: 16–352a (abs. 1259)
9. du Bois A., Richter B., et al. CisplatinPaclitaxel vs CarboplatinPaclitaxel as 1–ts–line treatment in OC. Proc. ASCO, 1998, 17, (abs. 1395).
10. Colombo N., et.al. Randomized trial of paclitaxel and carboplatin vs a control arm of carboplatin or CAP: the trial International collaborative Ovarian Neoplasm Study (ICON3). Proc. ASCO 2000;19:379a (abs.1500).
11. Colombo N., et.al. ICON3., Lancet 2002.
12. Verweij J., Clavel M., Chevalier B. Paclitaxel (Taxol) and docetaxel (Taxotere): not simply two of a kind. Ann Oncol. 1994, 5:495–505.
13. Kaern J., Trope C., et al. A stage of weekly taxol in patients with recurrent platinum resistant OC. 25–TH ESMO Congress. 13–17 Oct. 2000. vol.11, suppl.4, p.85 abs. 379p.
14. Hansen SW, Anderson H., Boman K. Et al. Gemcitabine, Carboplatin and Paclitaxel as first–line treatment of ovarian cancer FIGO Stages IIB–IV. Proc. ASCO, 1999, v.18, a.1379.
15. Papadimitriou C., Colombo N., Ieda N., et. al. The addition of ifosfamide or epirubicin to paclitaxel/cisplatin regimen in epithelial OC: randomized phase II study. Proc. ASCO, 1999, abs.1396
16. Engelholn S., Hovarth G., et. al., Reverse–schedule oral topotecan, paclitaxel and carboplatin in primary advanced OC: A phase I dose–randing study. 25 ESMO Congress 13–17 Oct., 2000– Ann. Onc. V.11., suppl.4., p.81., abs.361o.
17. Herben VM, Panday VR, et.al. Phase I and pharmacologic study of the combination of paclitaxel, cisplatin and topotecan administered intravenously every 21 days as first–line therapy in patients with advanced OC. J. Clin. Oncol. 1999,17(3), p.747–755.
18. Bolis G., Scarfone G., Sciatta C., et al. A phasse II stage of Topotecan, Carboplatin and Paclitaxel as front line treatment in suboptimal advanced epithelial ovarian cancer (AEOC). Proc. ASCO 2000, abs.1543.
19. Papadimitriou Ch., Gergoulias V., et.al., First–line treatment of advanced, suboptimally debulked, epithelial OC with the combination of ifosfamide, paclitaxel and cisplatin: long–term results of a phase II stage. 25 ESMO Congress 13–17 Oct., 2000– Ann. Onc. V.11., suppl.4., p.83., abs.371p.
20. Geertsen P., Hansen M., StrOyer J., et al. Combination chemotherapy with relapsed ovarian carcinoma. Proc. ASCO 1999, abs.1395.
21. Frickhofen N., Bunjes D., Berdel W. Et al. Two years of the German multicenter phase II trial of high-dose chemotherapy with stem cell support in advanced ovarian cancer. 25th ESMO Congress. –Annals of Onc., vol.11, suppl.4,Oct.2000,p.82,abs.363o.
22. Emelyanov D.E., Freichko N.V. Experience of using the drug Mitotax in the Sverdlovsk Regional Oncology Clinic of the Moscow Scientific and Practical Center “Oncology”., Healthcare of the Urals 2002 No. 8(14), pp. 33–34.
Similar drugs:
- Metoject Solution for injection
- Wartec Cream for external use
- Letrozole Oral tablets
- Medroxyprogesterone acetate Substance-powder
- Imuran Oral tablets
- Zoladex Capsule
- Paclitaxel Substance-powder
- Lomustine Oral tablets
- Votrient Oral tablets
- Cyclophosphan Substance-powder
** The Drug Directory is intended for informational purposes only. For more complete information, please refer to the manufacturer's instructions. Do not self-medicate; Before you start using Paclitaxel, you should consult your doctor. EUROLAB is not responsible for the consequences caused by the use of information posted on the portal. Any information on the site does not replace medical advice and cannot serve as a guarantee of the positive effect of the drug.
Are you interested in the drug Paclitaxel? Do you want to know more detailed information or do you need a doctor's examination? Or do you need an inspection? You can make an appointment with a doctor - the Euro lab is always at your service! The best doctors will examine you, advise you, provide the necessary assistance and make a diagnosis. You can also call a doctor at home . Euro lab clinic is open for you around the clock.
** Attention! The information presented in this medication guide is intended for medical professionals and should not be used as a basis for self-medication. The description of the drug Paclitaxel is provided for informational purposes only and is not intended for prescribing treatment without the participation of a physician. Patients need to consult a specialist!
If you are interested in any other drugs and medications, their descriptions and instructions for use, information about the composition and form of release, indications for use and side effects, methods of use, prices and reviews of drugs, or you have any other questions and suggestions - write to us, we will definitely try to help you.