Instructions for use PARIET
Antiulcer drug. H+-K+-ATPase (proton pump) inhibitor.
Mechanism of action
Rabeprazole sodium belongs to a class of antisecretory compounds that are chemically substituted benzimidazoles. The drug inhibits the activity of the enzyme H+-K+-ATPase, thereby blocking the final stage of hydrochloric acid synthesis. This effect is dose-dependent and leads to inhibition of both basal and stimulated acid secretion, regardless of the stimulus. As a weak base, rabeprazole in any dose is quickly absorbed and concentrated in the acidic environment of parietal cells.
Antisecretory activity
After oral administration of rabeprazole at a dose of 20 mg, the antisecretory effect occurs within 1 hour and reaches a maximum after 2-4 hours. Inhibition of basal and food-stimulated acid secretion 23 hours after taking the first dose of rabeprazole sodium was 62 and 82%, respectively, and the duration of this the effect reached 48 hours. The inhibitory effect of rabeprazole sodium on acid secretion increases slightly with daily intake of 1 tablet, stable inhibition of secretion is achieved 3 days after starting the drug. After stopping taking rabeprazole, secretory activity is restored after 2-3 days.
The role of Helicobacter pylori in the development of peptic ulcers, including gastric ulcers and duodenal ulcers, has been noted. Helicobacter pylori is a major factor in the development of gastritis and ulcers in such patients. Recent studies suggest a causal relationship between Helicobacter pylori and gastric carcinoma.
In vitro, rabeprazole was found to have a bactericidal effect against Helicobacter pylori. Eradication of Helicobacter pylori with Pariet and antimicrobial drugs leads to a high degree of healing of mucosal lesions. Based on the results of controlled randomized clinical trials, it was found that taking rabeprazole 20 mg 2 times a day in combination with two antibiotics, for example, clarithromycin and amoxicillin or clarithromycin and metronidazole for 1 week, can achieve a Helicobacter pylori eradication rate of more than 80% in patients with gastroduodenal ulcers. As expected, a trend toward lower eradication rates was observed in patients with underlying metronidazole resistance. When choosing an appropriate combination for Helicobacter pylori eradication, one should be guided by approved treatment standards. In patients with persistent infection (in the presence of initially sensitive strains of microorganisms), it is necessary to take into account the possibility of developing secondary resistance to antibacterial drugs when choosing a treatment regimen.
Effect on serum gastrin concentrations
During clinical trials, patients took 10 mg or 20 mg of rabeprazole once a day for a treatment duration of up to 43 months. During the first 2-8 weeks of therapy, serum gastrin concentrations increased, reflecting an inhibitory effect on acid secretion. Gastrin concentrations returned to baseline levels usually within 1-2 weeks after cessation of treatment.
A study of biopsies of the fundus and antrum of the stomach in more than 500 patients treated with rabeprazole for up to 8 weeks did not reveal changes in the histology of enterochromaffin-like cells, the severity of gastritis, the incidence of atrophic gastritis, intestinal metaplasia and the prevalence of Helicobacter pylori infection. In a study of 250 patients taking rabeprazole for 36 months, no significant deviations from the baseline were found.
Other effects
There is currently no evidence that rabeprazole causes systemic effects on the central nervous system, cardiovascular and respiratory systems. Rabeprazole, when taken orally at a dose of 20 mg/day for 2 weeks, had no effect on thyroid function, carbohydrate metabolism, as well as on blood concentrations of parathyroid hormone, cortisol, estrogen, testosterone, prolactin, cholecystokinin, secretin, glucagon, FSH, LH, renin, aldosterone and growth hormone.
Pariet, 10 mg, enteric-coated tablets, 7 pcs.
Pharmacodynamics
A drug that reduces the secretion of gastric glands - a proton pump inhibitor.
Mechanism of action
Rabeprazole sodium belongs to the class of antisecretory substances, benzimidazole derivatives. Rabeprazole sodium suppresses gastric acid secretion by specifically inhibiting H+/K+ ATPase on the secretory surface of gastric parietal cells. H+/K+ ATPase is a protein complex that functions as a proton pump, thus rabeprazole sodium is an inhibitor of the proton pump in the stomach and blocks the final stage of acid production. This effect is dose-dependent and leads to suppression of both basal and stimulated acid secretion, regardless of the stimulus. Rabeprazole sodium does not have anticholinergic properties.
Antisecretory action
After oral administration of 20 mg of rabeprazole sodium, the antisecretory effect develops within an hour. Inhibition of basal and stimulated acid secretion 23 hours after taking the first dose of rabeprazole sodium is 69% and 82%, respectively, and lasts up to 48 hours. This duration of pharmacodynamic action far exceeds that predicted by T1/2 (approximately one hour). This effect can be explained by prolonged binding of the drug to the H+/K+ ATPase of gastric parietal cells. The magnitude of the inhibitory effect of rabeprazole sodium on acid secretion reaches a plateau after three days of taking rabeprazole sodium. When you stop taking it, secretory activity is restored within 1-2 days.
Effect on plasma gastrin levels
In clinical studies, patients took 10 or 20 mg of rabeprazole sodium daily for treatment durations of up to 43 months. Plasma gastrin levels were elevated in the first 2–8 weeks, reflecting an inhibitory effect on acid secretion. Gastrin concentrations returned to baseline levels usually within 1-2 weeks after cessation of treatment.
Effect on enterochromaffin-like cells
In a study of human gastric biopsy specimens from the antrum and fundus of the stomach of 500 patients treated with rabeprazole sodium or a comparator for 8 weeks, persistent changes in the morphological structure of enterochromaffin-like cells, severity of gastritis, incidence of atrophic gastritis, intestinal metaplasia, or prevalence of Helicobacter infection pylori were not detected.
In a study of more than 400 patients treated with rabeprazole sodium (10 mg/day or 20 mg/day) for up to 1 year, the incidence of hyperplasia was low and comparable to that of omeprazole (20 mg/kg). There have been no reported cases of adenomatous changes or carcinoid tumors observed in rats.
Other effects
Systemic effects of rabeprazole sodium on the central nervous system, cardiovascular or respiratory systems have not currently been detected. It has been shown that rabeprazole sodium, when taken orally at a dose of 20 mg for 2 weeks, has no effect on thyroid function, carbohydrate metabolism, the level of parathyroid hormone in the blood, as well as on the levels of cortisol, estrogens, testosterone, prolactin, glucagon, follicle-stimulating hormone (FSH), luteinizing hormone (LH), renin, aldosterone and growth hormone.
Pharmacokinetics
Suction
Rabeprazole is rapidly absorbed from the intestine and peak plasma concentrations are achieved approximately 3.5 hours after a 20 mg dose. Changes in peak plasma concentrations (Cmax) and area under the concentration-time curve (AUC) of rabeprazole are linear over the dose range from 10 to 40 mg. Absolute bioavailability after oral administration of 20 mg (compared to intravenous administration) is approximately 52%. In addition, bioavailability does not change with repeated dosing of rabeprazole. In healthy volunteers, the plasma T1/2 period is about 1 hour (ranging from 0.7 to 1.5 hours), and the total clearance is 3.8 ml/min/kg.
In patients with chronic liver damage, AUC is doubled compared to healthy volunteers, indicating a decrease in first-pass metabolism, and T1/2 from plasma is increased by 2-3 times. Neither the time of taking the drug during the day nor antacids affect the absorption of rabeprazole. Taking the drug with fatty foods slows down the absorption of rabeprazole by 4 hours or more, but neither Cmax nor the degree of absorption changes.
Distribution
In humans, the degree of binding of rabeprazole to plasma proteins is about 97%.
Metabolism and excretion
In healthy people
After taking a single oral dose of 20 mg of 14C-labeled rabeprazole sodium, no unchanged drug was found in the urine. About 90% of rabeprazole is excreted in the urine mainly in the form of two metabolites: a conjugate of mercapturic acid (M5) and carboxylic acid (M6), as well as in the form of two unknown metabolites identified during toxicological analysis. The remainder of the rabeprazole sodium taken is excreted in the feces. The total elimination is 99.8%. These data indicate a small excretion of rabeprazole sodium metabolites in bile. The main metabolite is thioester (M1). The only active metabolite is desmethyl (M3), but this was observed at low concentrations in only one study participant after taking 80 mg rabeprazole.
End stage renal failure
In patients with stable end-stage renal disease who require maintenance hemodialysis (creatinine clearance <5 ml/min/1.73 m2), the elimination of rabeprazole sodium is similar to that of healthy volunteers. AUC and Cmax in these patients were approximately 35% lower than in healthy volunteers. On average, T1/2 of rabeprazole was 0.82 hours in healthy volunteers, 0.95 hours in patients during hemodialysis and 3.6 hours after hemodialysis. Clearance of the drug in patients with kidney disease requiring hemodialysis was approximately twice as high as in healthy volunteers.
Chronic compensated cirrhosis
Patients with chronic compensated liver cirrhosis tolerate rabeprazole sodium 20 mg once daily, although AUC is doubled and Cmax is increased by 50% compared to sex-matched healthy volunteers. Elderly patients In elderly patients, the elimination of rabeprazole is somewhat slower. After 7 days of rabeprazole 20 mg per day in elderly subjects, the AUC was approximately twice as high and the Cmax was increased by 60% compared to young healthy volunteers. However, there were no signs of accumulation of rabeprazole.
CYP2C19 polymorphism
In patients with slow metabolism of CYP2C19, after 7 days of taking rabeprazole at a dose of 20 mg per day, AUC increases by 1.9 times and Cmax by 1.6 times compared with the same parameters in “rapid metabolizers,” while Cmax increases by 40%.
Pariet®
Cytochrome 450 system
Rabeprazole sodium, like other proton pump inhibitors (PPIs), is metabolized by the cytochrome P450 (CYP450) system in the liver. In vitro studies on human liver microsomes have shown that rabeprazole sodium is metabolized by the isoenzymes CYP2C19 and CYP3A4.
Studies in healthy volunteers have shown that rabeprazole sodium has no pharmacokinetic or clinically significant interactions with drugs that are metabolized by the cytochrome P450 system - warfarin, phenytoin, theophylline and diazepam (regardless of whether patients metabolize diazepam extensively or poorly).
A study of combination therapy with antibacterial drugs was conducted.
This four-way crossover study involved 16 healthy volunteers who received rabeprazole 20 mg, amoxicillin 1000 mg, clarithromycin 500 mg, or a combination of these three drugs (RAC - rabeprazole, amoxicillin, clarithromycin). AUC and Cmax values for clarithromycin and amoxicillin were similar when combination therapy was compared with monotherapy. AUC and Cmax for rabeprazole increased by 11% and 34%, respectively, and for 14-hydroxy-clarithromycin (the active metabolite of clarithromycin), AUC and Cmax increased by 42% and 46%, respectively, for combination therapy compared with monotherapy. This increase in exposure rates for rabeprazole and clarithromycin was not considered clinically significant.
Interactions due to inhibition of gastric acid secretion
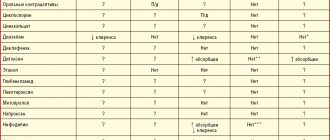
Rabeprazole sodium provides a stable and long-lasting suppression of gastric acid secretion. Thus, interactions may occur with substances for which absorption is pH dependent. When taken simultaneously with rabeprazole sodium, the absorption of ketoconazole is reduced by 30%, and the absorption of digoxin is increased by 22%. Therefore, some patients should be monitored to determine whether dose adjustments are necessary when taking rabeprazole sodium concomitantly with ketoconazole, digoxin, or other drugs for which absorption is pH dependent.
Atazanavir
When atazanavir 300 mg/ritonavir 100 mg was coadministered with omeprazole (40 mg once daily) or atazanavir 400 mg with lansoprazole (60 mg once daily) in healthy volunteers, a significant reduction in atazanavir exposure was observed. Absorption of atazanavir is pH dependent. Although concomitant use with rabeprazole has not been studied, similar results are expected for other proton pump inhibitors. Therefore, concomitant use of atazanavir with proton pump inhibitors, including rabeprazole, is not recommended.
Antacids
In clinical studies, antacid substances were used in conjunction with rabeprazole sodium. Clinically significant interactions of rabeprazole sodium with aluminum hydroxide gel or magnesium hydroxide were not observed.
Eating
In a clinical study, no clinically significant interactions were observed when rabeprazole sodium was taken with a low-fat meal. Taking rabeprazole sodium simultaneously with a fat-enriched meal may slow down the absorption of rabeprazole by up to 4 hours or more, but Cmax and AUC do not change.
Cyclosporine
In vitro experiments using human liver microsomes showed that rabeprazole inhibits the metabolism of cyclosporine with an IC50 of 62 μmol, i.e., at a concentration 50 times the Cmax for healthy volunteers after 14 days of administration of 20 mg rabeprazole. The degree of inhibition is similar to that of omeprazole for equivalent concentrations.
Methotrexate
Adverse event reports, published pharmacokinetic studies, and retrospective analyzes suggest that concomitant use of PPIs and methotrexate (primarily at high doses) may result in increased concentrations of methotrexate and/or its metabolite hydroxymethotrexate and prolong the elimination half-life. However, no specific drug interaction studies have been conducted between methotrexate and PPIs.
Impact on laboratory results
The use of PPIs leads to a decrease in gastric acidity, which can lead to an increase in serum chromogranin A (CgA). Elevated CgA levels may lead to misinterpretation of laboratory results for the presence of a neuroendocrine tumor. To avoid this effect, the use of Pariet® should be temporarily discontinued at least 14 days before assessing CgA levels; repeating the test should be considered if the initial CgA level is high.