Standard pharmacopoeial solutions (liquids) are aqueous solutions (factory production) of certain medicinal substances in a strictly defined concentration, specified in the relevant articles of the Global Fund.
These include solutions of solid, liquid or gaseous substances:
- potassium acetate solution,
- Burov's fluid,
- hydrochloric acid,
- ammonia solution,
- hydrogen peroxide,
- formalin, etc.
These liquids are easily mixed with water, and their solutions are prepared directly in the dispensing bottle, into which the water is first measured, and then the calculated amount of liquid. If necessary, filter the solution.
Rules for prescribing standard pharmacopoeial solutions
Standard pharmacopoeial solutions can be prescribed under two names: conditional and chemical (see table below), on which the calculation of their quantity depends.
NB!!!! If in the prescription the liquid is prescribed under a code name, then in the calculations the concentration of the standard solution is taken as one (100%).
NB!!!! If the chemical name is indicated, then the calculations are based on the actual content of substances in standard solutions, using the following formula:
X = V * (V/A)
where X is the volume of standard liquid, ml;
V is the volume of solution to be prepared, ml;
B is the prescribed concentration of the solution, %;
A is the actual concentration of the standard liquid to be diluted, %.
| Code name | Chemical name | Concentration, % | Literary |
| Burov's fluid | Aluminum acetate solution basic | 7,6—9,2 | GF IX |
| Potassium acetate liquid | Potassium acetate solution | 33—35 | GF VIII |
| Formalin | Formaldehyde solution | 36,5—37,5 | GF X |
| Perhydrol | Concentrated hydrogen peroxide solution | 27,5—31,0 | GF X |
| Hydrogen peroxide solution | 2,7—3,3 | GF X | |
| Ammonia solution | Ammonia solution | 9,5—10,5 | GF IX |
| Acetic acid | Acetic acid | 3; 29,5—30,5; 98 | GF VII |
Hydrochloric acid | Hydrochloric acid | 24,8—25,2 | GF X |
| Hydrochloric acid, diluted | Hydrochloric acid, diluted | 8,2—8,4 | GF X |
The amount of water in both cases is calculated by the difference between the total volume of the prepared solution and the calculated amount of standard liquid.
Why is the drug prescribed?
According to the designation of the European Pharmacopoeia, hydrogen peroxide is called hydrogen peroxide. In Latin, hydrogen peroxide sounds like Hydrogenii peroxydi dilutae - it is by this name that the doctor writes a prescription.
Please note that you cannot purchase hydrogen peroxide in European pharmacy chains without a doctor’s prescription, despite the high safety of the medicine. This practice applies to all medications - prescriptions for them must be issued by a doctor.
The drug is prescribed for rinsing and washing for diseases of the mouth and throat, and gynecological pathologies. The antiseptic is effective for treating shallow wounds and against nosebleeds.
Formaldehyde solution (formalin)
Used as a disinfectant and deodorant:
- for washing hands,
- washing the skin with excessive sweating (0.5-1% solutions),
- for disinfection of instruments (0.5% solution),
- for douching 1:2000—1:3000.
Example No. 3
Rp.: Solutionis Formalini 5% 100 ml
Yes. Signa. For disinfection of premises
The solution was prescribed under a code name.
Calculation: Formaldehyde solution 36.5-37.5%
X = 5*100/100 = 5 ml
Purified water : 100 – 5 = 95 ml
Manufacturing technology: 95 ml of purified water and 5 ml of a standard formaldehyde solution are measured into an orange glass dispensing bottle. Getting ready for vacation.
Example No. 4
Rp .: Solutionis Formaldegydi 10% 100 ml
Da . Signa . 1 teaspoon per glass of water to wash your feet
In this case, a solution of formaldehyde under the chemical name is prescribed.
Calculation: 37% formaldehyde solution X = 10 * 100/37 = 27 ml
Purified water : 100 – 27 = 73 ml
Burov's liquid solution
It has an astringent and local anti-inflammatory effect, and in high concentrations it has moderate antiseptic properties. It is used diluted (10-20 times or more) for:
- rinsing,
- lotions,
- douching,
- for inflammatory diseases of the skin and mucous membranes.
Example No. 5
Rp.: Solutionis Liquoris Burovi 10% 100 ml
Yes. Signa. Lotion
In this case, the standard liquid is prescribed under a code name. The volume of the solution is 100 ml. 90 ml of purified water and 10 ml of an 8% solution of basic acetic-aluminum salt are measured into the bottle and prepared for release.
If a solution of basic aluminum acetate (chemical name) is prescribed, then the calculations are based on its actual content in Burov’s liquid, that is, 8%.
Example No. 6
Rp.: Solutionis Aluminii subacetatis 0.8% 100 ml
Yes. Signa. Lotion
In this case, the calculation is made using the formula:
X =0.8/8*100= 10 ml of 8% basic solution of aluminum acetate
Volume of purified water: 100-10 = 90ml
Potassium acetate solution
This is a solution of potassium carbonate or bicarbonate in dilute acetic acid. Used as a diuretic for edema associated with poor circulation.
There are variants of writing:
a) Rp.: Liquoris Kalii acetatis 10% 200 ml
b) Rp.: Liquoris Kalii acetatis ex 20.0 200 ml
c) Rp.: Solutionis liquoris Kalii acetatis 10% 200 ml
The drug according to all prescriptions is prepared from a solution of potassium acetate, taking it as a unit (100%), that is, take 20 ml of pharmacopoeial liquid and 180 ml of purified water.
Example No. 7
Rp.: Solutionis Kalii acetatis 10% 200 ml
Yes. Signa. 1 tablespoon 4 times a day
In this case, the solution is prescribed under a chemical name, so the calculation is carried out using the above formula:
Potassium acetate solution
X =10*200/34 = 58, 8 or 59 ml
Purified water 200 – 59 = 141 ml
Hydrochloric acid solution
Intended mainly for internal use in the form of drops and mixtures in case of insufficient acidity of gastric juice. Considering that it is prescribed to both adults and children, the methods of prescription and the concentration of hydrochloric acid may be different. Therefore, calculations associated with the preparation of these solutions require special attention.
In all cases where hydrochloric acid is prescribed without indicating the concentration, diluted hydrochloric acid (Acidum hydrochloricum dilutum 8.3%) is dispensed. Dispense as much as prescribed in the prescription.
Example No. 8
Rp .: Acidi hydrochlorici 4 ml
Aquae purificatae 200 ml
Misce. Yes. Signa. 1 tablespoon 3 times a day before meals
Measure 200 ml of purified water into the dispensing bottle, then 4 ml of diluted hydrochloric acid 8.3% and shake until the liquids are completely mixed.
The preparation of hydrochloric acid solutions for dispensing does not differ in any particular way.
If a solution of hydrochloric acid (with a concentration designation) is prescribed for internal use, then diluted hydrochloric acid (8.3%) is used to prepare it, taking it as a unit (100%) for calculations.
Example No. 9
Rp.: Solutionis Acidi hydrochlorici 2% 100 ml
Da . Signa . 1 teaspoon 3 times a day before meals
Calculation: Diluted hydrochloric acid 2 ml
Purified water 100 – 2 = 98 ml
However, given the volatility of hydrogen chloride, in order to increase the accuracy of the prepared solutions, it is recommended to use a pre-prepared dilution of this acid (in-pharmacy preparation) Solutio Acidi hydrochlorici diluti (1:10), which contains 0.83% hydrogen chloride.
A solution of diluted hydrochloric acid (1:10) is prepared by diluting hydrochloric acid (8.3%) with an appropriate amount of water. For example, to prepare 1 liter of solution, you need to take 900 ml of purified water and add 100 ml of hydrochloric acid (8.3%).
This solution is taken 10 times more than the prescribed amount of acid in the recipe.
Calculation: Diluted hydrochloric acid solution (1:10) 2•10 = 20 ml
Purified water 100 – 20 = 80 ml
To prepare the mixture according to the above recipe, measure 80 ml of purified water and 20 ml of diluted hydrochloric acid solution (1:10) into a dispensing bottle.
Hydrochloric acid (24.8-25.2%) is used in pharmacies as a reagent, and it is also used for external purposes in the preparation of Demyanovich’s liquid (author’s recipe), taking it as a unit in calculations.
Demyanovich's liquid consists of two solutions intended for the treatment of patients with scabies.
Example No. 10
Rp.: Solutionis Natrii thiosulfatis 60% 100 ml
Yes. Signa. External ( Solution No. 1 )
AND
Rp.: Solutionis Acidi hydrochlorici 6% 100 ml
Yes. Signa. External (Solution No. 2)
The combined use of two solutions is based on the ability of sodium thiosulfate to decompose in an acidic environment, releasing sulfur and sulfur dioxide, which have an antiparasitic effect.
Preparation of solution No. 1:
The recipe for this solution is the author’s, so it is prepared by weight (60.0 g + 40.0 g) = 100.0 g. If it is necessary to prepare 100 ml of a solution in mass-volume concentration, certain calculations should be made. 100.0 g of 60% sodium thiosulfate solution takes up a volume of 73.5 ml, so to prepare 100 ml of solution you should take 81.63 g of sodium thiosulfate: NB !!!!
60.0 - 73.5 ml
x - 100 ml
X = 81.63 g
prepared taking into account the CV of sodium thiosulfate: 100 - (81.63•0.51) = 58 ml. NB !!!!
It is prohibited to prepare a solution by dissolving 60.0 g of sodium thiosulfate and bringing the resulting solution to a volume of 100 ml, since the mass-volume concentration of the drug in the solution will be only 46.37%. NB !!!!
To prepare solution No. 2, hydrochloric acid 24.8-25.2% should take 6 ml. Then diluted hydrochloric acid (8.3%) must be taken 3 times more (see GF X, article 18, p. 55), that is, 18 ml, and water, respectively, 82 ml. NB !!!!
Obtaining H2O2.
H2O2 molecules are always produced in small quantities during the combustion and oxidation of various compounds. During combustion, H2O2 is formed either by the abstraction of hydrogen atoms from the starting compounds by intermediate hydroperoxide radicals, for example: HO2 . + CH4 ® H2O2 + CH3 . , or as a result of recombination of active free radicals: 2OH . ® H2O2, N . + HO2 . ® H2O2. For example, if an oxygen-hydrogen flame is directed at a piece of ice, then the melted water will contain noticeable quantities of H2O2 formed as a result of the recombination of free radicals (H2O2 molecules immediately disintegrate in the flame). A similar result is obtained when other gases burn. The formation of H2O2 can also occur at low temperatures as a result of various redox processes.
In industry, hydrogen peroxide has long been no longer produced using the Tenara method - from barium peroxide, but more modern methods are used. One of them is electrolysis of sulfuric acid solutions. In this case, at the anode, sulfate ions are oxidized to persulfate ions: 2SO42– – 2e ® S2O82–. The persulfuric acid is then hydrolyzed:
H2S2O8 + 2H2O ® H2O2 + 2H2SO4.
At the cathode, as usual, hydrogen evolution occurs, so the overall reaction is described by the equation 2H2O ® H2O2 + H2. But the main modern method (over 80% of world production) is the oxidation of some organic compounds, for example, ethylanthrahydroquinone, with atmospheric oxygen in an organic solvent, while H2O2 and the corresponding anthraquinone are formed from anthrahydroquinone, which are then reduced again with hydrogen on a catalyst into anthrahydroquinone. Hydrogen peroxide is removed from the mixture with water and concentrated by distillation. A similar reaction occurs when isopropyl alcohol is used (it occurs with the intermediate formation of hydroperoxide): (CH3)2CHOH + O2 ® (CH3)2C(OOH)OH ® (CH3)2CO + H2O2. If necessary, the resulting acetone can also be reduced to isopropyl alcohol.
Acetic acid solution.
The original acetic acid can be diluted (29.5-30.5%) or concentrated (98%).
In medical practice, 5-8% solutions of acetic acid are used for external use (wiping). When preparing these solutions, they always proceed from its actual content in the original solution.
NB!!!!If the acid concentration is not indicated in the recipe, then prepare a 30% solution of acetic acid.
Example No. 11
Rp .: Solutionis Acidi acetici 5% 100 ml
Da . Signa . For wiping
To prepare this solution, it is better to use diluted acetic acid.
Calculation: diluted acetic acid
X = 5*100/30= 17 ml
Purified water 100 – 17 = 83 ml
Measure 83 ml of water into the dispensing bottle and add 17 ml of diluted acetic acid.
Ammonia solution
It is a 10% solution of ammonia in water, which is used as an ambulance to induce breathing and bring patients out of fainting. Sometimes used internally as an emetic (5-10 drops per 100 ml of water); for insect bites, apply externally in the form of lotions; in surgical practice - for washing hands (25 ml per 5 liters of warm boiled water).
If an ammonia solution is prescribed without indicating the concentration, then a pharmacopoeial preparation containing 10% ammonia (Solutio Ammonii caustici) - ammonia - is always meant.
When preparing ammonia solutions of the concentration required by recipe, calculations are always based on the actual ammonia content in the solution.
Example No. 12
Rp.: Solutionis Ammonii caustici 0.5% 500 ml
Yes. Signa. Hand washing
Calculation: 10% ammonia solution
X= 0.5*500/10= 25 ml
Purified water: 500 – 25 = 475 ml
To prepare a solution according to this recipe, measure 475 ml of purified water and 25 ml of a 10% ammonia solution into a dispensing bottle.
The concentration of ammonia in the solution changes, so it is necessary to periodically monitor it, and if necessary, strengthen the solution by adding a stronger 25-27% ammonia solution (GF X, p. 868). When mixing liquids, use the following formulas:
NB !!!! X = M * (a – c)/(B – c)
NB !!!! Y = M - X
NB !!!! where X is the amount of strong solution that must be used to strengthen the weak solution, ml;
M is the amount of solution that needs to be prepared, ml;
a is the desired strength of this solution, %;
b is the concentration of the available strong solution, %;
c is the concentration of the available weak solution, %;
Y is the amount of available weak solution, ml.
For example, it is necessary to prepare 10 liters of a 10% ammonia solution from an existing 5% solution by mixing it with a 25% ammonia solution:
X =10 * (10 – 5)/ (25-5) = 2.5 l;
Y = 10 − 2.5 = 7.5 l.
Thus, to obtain 10 liters of a 10% ammonia solution, you need to take 7.5 liters of a 5% solution and 2.5 liters of a 25% ammonia solution.
Hydrogen peroxide - benefits for the body
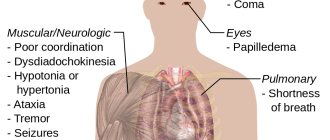
Treatment with hydrogen peroxide is a new universal preventive remedy, thanks to which you can avoid strokes, heart attacks, eliminate atherosclerosis and acute vascular diseases. After Professor Neumyvakin studied hydrogen peroxide (H2O2), using it on himself and experiencing all its properties, the drug began to be widely used.
At first, hydrogen peroxide was used only against cardiovascular diseases, but then it was discovered that it could also be used to treat allergies, bronchitis and emphysema. The drug has properties that destroy cancer cells, remove fats from the walls of blood vessels and treat leukemia. There are no contraindications to the drug, you just need to calculate the dosage correctly.
Hydrogen peroxide is used for various infections and diseases; it protects well against viruses, bacteria and fungi. Thanks to this drug, you can stimulate the immune system, and it is also a universal remedy for supporting vital processes and with its help the body resists against any diseases and infections.
When hydrogen peroxide enters the body, it decomposes into water (H2O) and atomic oxygen (O). And it is he who enters the fight for human life, but only if used correctly. Treatment with hydrogen peroxide is especially effective in the presence of vitamin C, so during this treatment it is recommended to take these vitamins in pill form or eat foods rich in vitamin C.
It is important to remember that peroxide can only be taken on an empty stomach, respectively, half an hour before meals or no earlier than 2 hours after meals. Important note: before starting treatment with hydrogen peroxide, you should cleanse your body of toxins: cleanse the liver, joints, blood, kidneys.
Hydrogen peroxide-based preparations can be taken together with various herbal remedies, but cannot be combined with medications. Medicines can only be used half an hour after H2O2 treatment. When taking the medicine, you should not worry about a slight burning sensation in the stomach; if it appears, you need to reduce the dose of the drug until the body gets used to it.
Non-aqueous solutions
In medical practice, solutions based on non-aqueous solvents (non-aqueous solutions) are widely used as:
- lotions,
- rinsing,
- lubrication,
- washings,
- intranasal drops,
- inhalations.
Depending on the properties of the solvent, non-aqueous solutions using volatile, non-volatile and combined solvents are distinguished.
Volatile liquids that are used as solvents include:
- ethanol,
- chloroform,
- ether.
To non-volatile:
- glycerol,
- fatty oils (peach, almond, sunflower),
- Vaseline oil,
- dimexide.
Preparation of solutions using volatile solvents.
In this case, it is necessary to take into account the possibility of significant losses of solvent and a corresponding increase in the concentration of the solution due to evaporation during the preparation process.
To avoid these losses, operations such as heating, filtering or straining are undesirable. In addition, ethyl alcohol and ether, with the exception of chloroform, are flammable, so dissolution in this case should be carried out while observing safety precautions (away from fire).
NB !!!! Alcohol, ether and chloroform solutions are prepared directly in dispensing bottles. Vials must be clean and dry, since water does not mix well with organic solvents (except alcohol) and changes their dissolving ability.
NB !!!! When preparing alcohol solutions, unlike aqueous ones, first the dissolving drug substance is placed in a dry dispensing bottle (if it is bulky and loose, then use a dry funnel), and then the solvent, since pouring the powder through the neck of the bottle moistened with alcohol is difficult.
Straining these solutions is carried out, if necessary, through a small lump of dry cotton wool using a funnel covered with glass. Straining ethereal solutions is especially undesirable.
Ethyl alcohol is most often used as a volatile solvent in pharmacy practice.
Alcohol solutions.
Ethyl alcohol and its aqueous solutions are used to dissolve many medicinal substances:
- organic acids,
- alkaloid bases,
- essential oils,
- iodine,
- camphor,
- resorcinol,
- menthol,
- hydrogen peroxide,
- formaldehyde and other substances.
Ethyl alcohol can also be used as a medicine that has disinfectant, refreshing and irritant properties, for compresses, etc.
NB!!!!If the recipe does not indicate the concentration of ethyl alcohol, then use 90%.
NB !!!! The exception is a 10% iodine solution, which is prepared using 95% alcohol according to the recipe of the State Fund X Art. 356, as well as some solutions, according to the approved regulatory and technical documentation
If the strength of ethyl alcohol is indicated as a percentage, the percentage by volume should be understood.
Example No. 13
Rp.: Acidi salicylici 0.3
Spiritus aethylici 30 ml
Misce. Yes. Signa. Wipe your feet
NB !!!! The prescription must be issued with the stamp of the medical institution, the personal seal and signature of the doctor, and the seal of the medical institution “For prescriptions”.
NB !!!! To prepare a 1% solution of salicylic acid, use 70% alcohol.
Place 0.3 g of salicylic acid in a clean, dry bottle with a well-selected stopper, measure out 30 ml of 70% ethyl alcohol with a measuring cylinder and quickly close the stopper to prevent the alcohol from evaporating. The medicinal product is issued with a signature .
If the pharmacy does not have ready-made 70% alcohol, then it is prepared from the alcohol of the available concentration.
Rules for diluting alcohol
Diluting ethyl alcohol with water to the required concentration requires appropriate calculations. To do this, use the alcoholometric tables of GF XI of alcohol of various strengths at 20 ° C, see order No. 308 - a table showing the amount (in ml at 20 ° C) of water and alcohol of various strengths that must be mixed to obtain 1 liter of alcohol with a strength of 30 , 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90%.
As a rule, pharmacies receive alcohol with an anhydrous alcohol content of over 96% (96.1-96.7). Therefore, to prepare standard water-alcohol solutions, the existing strong alcohol is diluted using table No. 5 (GF XI), which indicates (in ml at 20 ° C) the amount of water and alcohol of various strengths (95.1-96.5), which must be mixed to obtain 1 liter (at 20°C) of alcohol with a strength of 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95%.
In the order of the Ministry of Health No. 308, tables are given that indicate the correspondence of volumes (ml) of ethyl alcohol of various concentrations to the mass (g) of alcohol (at 20 ° C).
The concentration of the original alcohol is given in percent by volume for 95, 96, 96.1, 96.2, 96.3, 96.4, 96.5, 96.6, 96.7%.
The dilution formula is also used for calculations:
X =V*B/A
where X is the amount of strong alcohol, ml;
V is the amount of ethyl alcohol of the required concentration, ml;
A is the concentration of strong alcohol, ml;
B is the required concentration, %.
To prepare 70% alcohol according to the above recipe, the amount of 90% alcohol is 23.3 ml:
X =30 * 70/90= 23.3 ml.
The amount of water required to prepare 70% alcohol cannot be calculated by subtracting the volume of 90% alcohol from the total volume of the solution, since contraction - a decrease in volume - must be taken into account.
The found amount of 90% alcohol (23.3 ml) is measured with a measuring cylinder at 20 °C, approximately 7 ml of water is added, the solution is stirred and cooled to 20 °C, and then diluted with water to the required volume of 30 ml.
Using table No. 4 (GF XI), you can calculate the amount of both components:
Alcohol 90% - 23.34 ml
Water - 7.2 ml
NB !!!! Since subject-quantitative accounting in pharmacies is carried out by weight, at the same time they recalculate volume units into weight units.
Example No. 14
Rp.: Acidi salicylici 1.5
Laevomycetini 3.0
Camphorae 1.0
Sp. aethylici 50 ml
Tincturae Calendulae 10 ml
Misce. Yes. Signa. Wipe your face
Place 3.0 g of chloramphenicol, 1.5 g of salicylic acid, 1.0 g of camphor into the dispensing bottle, add 50 ml of 90% ethyl alcohol and shake. After the substances have dissolved, add 10 ml of calendula tincture.
Preparation of solutions using non-volatile solvents.
Solutions of medicinal substances in non-volatile solvents are prepared by weight, since the significant viscosity of these solvents leads to large losses during measuring.
The mass of such solutions is the sum of the quantities of medicinal substances and solvent.
Considering that dissolution in viscous solvents proceeds slowly, it is advisable to carry out it by heating, taking into account the properties of medicinal substances. However, in this case, it is necessary to avoid preparing saturated solutions, since when such a solution is cooled, the soluble substance may precipitate. Solutions with viscous solvents are prepared directly in bottles for dispensing, and are filtered only in extreme cases and only through gauze.
Glycerin solutions
Widely used as various lubricants. The following is prescribed in the form of glycerin solutions:
- boric acid,
- sodium tetraborate,
- iodine,
- tannin,
- ichthyol and other substances.
Glycerin has significant viscosity, so the preparation of glycerin solutions can occur with or without heating, which completely depends on the thermolability of the incoming medicinal substances. When heated to 40-50 °C, the viscosity of glycerin decreases and the dissolution process accelerates.
NB !!!! It is better to dissolve sodium tetraborate and boric acid in heated glycerin; when dissolved, they form glyceroboric acid, which gives the solutions an acidic reaction.
To neutralize glyceroboric acid, sodium bicarbonate is often prescribed along with boric acid. It should be added carefully in small portions, since the neutralization reaction proceeds violently and splashing of the solution may occur.
Example No. 15
Rp.: Acidi borici 1.0
Glycerini 90.0
Misce. Yes. Signa. For wetting tampons
Boric acid is placed in a dry dispensing bottle with a selected stopper, tared on a technical scale and 90.0 g of glycerin is weighed out, heated in a water bath at a temperature of 50-60 ° C until the boric acid is completely dissolved. Getting ready for vacation.
Example No. 16
Rp.: Natrii tetraboratis 1.0
Aquae purificatae
Glycerini aa - 5.0
Misce . Yes. Signa. Lubrication
The solubility of sodium tetraborate in water is 1:25, in glycerol 1:2.5. Therefore, 1.0 g of sodium tetraborate is placed in the dispensing bottle (through a dry funnel), tared (without a funnel) and glycerin is weighed into it, capped and heated in a water bath by immersing the bottle in warm water until the sodium tetraborate is completely dissolved. Then add 5 ml of purified water.
NB !!!! When preparing glycerin solutions of iodine, heating is undesirable.
Example No. 17
Rp.: Iodi 1.0
Kalii iodidi 2.0
Aquae purificatae 3 ml
Glycerini 94,0
Misce . Yes. Signa. For tampons for vulvovaginitis
First, prepare a concentrated solution of potassium iodide. Measure purified water into an orange glass dispensing bottle and dissolve potassium iodide in it, then iodine. The bottle is tared on technical pharmacy scales, glycerin is weighed out, shaken until a solution is obtained and prepared for dispensing.
If water is not prescribed in the recipe, it must be taken in a minimal amount (equal to the amount of potassium iodide).
Oily solutions.
Fatty oils, as well as petroleum jelly, are good solvents for many medications, which are quite widely used in the form of ear and intranasal drops.
To speed up dissolution, light heating is used.
NB !!!! If a volatile substance is prescribed in the oil solution, for example menthol, camphor, then to eliminate loss, dissolution is carried out in preheated oil at a temperature not exceeding 40 ° C.
Example No. 18
Rp.: Mentholi 0.1
Olei Vaselini 10.0
Misce. Yes. Signa. Nasal drops
10.0 g of vaseline oil is weighed into a dry bottle for dispensing, heated in a water bath to no higher than 40-50 ° C and then 0.1 g of menthol (odorous substance) is dissolved in it. Strain if necessary.
When preparing oil solutions, you need to pay attention to the preparation of ear drops with carbolic acid.
There are two phenol preparations in GF X: crystalline and liquid. If the recipe does not indicate which one to use, then take the crystalline one. Liquid phenol is used to prepare only aqueous solutions.
Example No. 19
Rp.: Acidi carbolici 0.4
Olei Helianthi 20.0
Misce. Yes. Signa. Ear drops
Place 0.4 g of crystalline phenol in a dry bottle for release, weighed on a hand scale on a mug made of parchment paper (being careful not to touch it with your hands to avoid burns).
The bottle is tared on a technical pharmacy scale, into which 20.0 g of sunflower oil is weighed, closed with a previously selected stopper with a gasket and shaken until the phenol is completely dissolved.
Non-aqueous solvents also include eutectic alloys, which are obtained by mutual dissolution of two solid substances having high cryoscopic constants or low melting points, or both.
Eutectic alloys are prepared by placing the prescribed medicinal substances in a dispensing bottle, which is well sealed with a stopper and placed in warm (40 ° C) water until they are completely melted.
When preparing significant quantities of liquid eutectic alloys, they sometimes resort to grinding and mixing in a mortar.
Example No. 20
Rp.: Camphorae
Chlorali hydrati a—a 1.5
Misce . Yes. Signa. Dental drops
Camphor and chloral hydrate are placed in a dry bottle for dispensing, tightly closed with a stopper, placed in warm (40 ° C) water and kept until completely melted - a liquid is formed.
Example No. 21
Rp.: Iodi 10.0
Dimexidi ad 100.0
Misce. Yes. Signa. Lubricate nails and feet
Place 10.0 g of iodine in a dry bottle for dispensing, tare the bottle and weigh out 90.0 g of dimexide and shake until dissolved (iodine solubility in dimexide is 1:1).
Chemical properties
Both oxygen atoms are in the intermediate oxidation state −1, which determines the ability of peroxides to act as both oxidizing agents and reducing agents. Their most characteristic oxidizing properties are:
Na2SO3 + H2O2 → Na2SO4 + H2O Mn(OH)2 + H2O2 → MnO(OH)2 + H2O
When interacting with strong oxidizing agents, hydrogen peroxide acts as a reducing agent, oxidizing to atomic oxygen:
2AgNO3 + H2O2 → 2Ag + 2O + 2HNO3
The hydrogen peroxide molecule is highly polar, which results in hydrogen bonds between the molecules. The O-O bond is weak, so H2O2 is an unstable compound and easily decomposes. The presence of transition metal ions may also contribute to this. In dilute solutions, hydrogen peroxide is also unstable and spontaneously disproportions into H2O and O. The disproportionation reaction is catalyzed by transition metal ions and some proteins:
2H2O2 → 2H2O + O2
However, very pure hydrogen peroxide is quite stable.
Hydrogen peroxide exhibits weak acidic properties (K = 1.4⋅10−12), and therefore dissociates in two steps:
H2O2 ⇄ H+ + HO2− ; HO2− ⇄ H+ + O22−
When a concentrated solution of H2O2 acts on some hydroxides, in some cases metal peroxides can be isolated, which can be considered as salts of hydrogen peroxide (Li2O2, MgO2, etc.):
H2O2 + 2NaOH → Na2O2 + 2H2O H2O2 + Ba(OH)2 → BaO2↓ + 2H2O
Hydrogen peroxide can exhibit both oxidizing and reducing properties. For example, when interacting with silver oxide, it is a reducing agent:
H2O2−1 + Ag2O ⟶ 2Ag + O20 + H2O
In the reaction with potassium nitrite, the compound serves as an oxidizing agent:
KNO2 + H2O2−1 ⟶ KNO3−2 + H2O
The peroxide group [—O—O—] is found in many substances. Such substances are called peroxides or peroxide compounds. These include metal peroxides (Na2O2, BaO2, etc.). Acids containing a peroxide group are called peroxoacids, for example, peroxomonophosphoric acid H3PO5, peroxodisulphuric acid H2S2O8 and peroxonitric acid HNO4.