Release form, packaging and composition of the drug Clinical-pharmacological group Pharmaco-therapeutic group Pharmacological action Indications for use Method of administration and doses Side effects Contraindications for use Use in children Special instructions Drug interactions
Registration Certificate Holder:
FRESENIUS KABI DEUTSCHLAND, GmbH (Germany)
ATX Code:
A11BA
Active substance:
Dosage form:
Soluvit N
| The drug is available with a prescription | Soluvit N | Lyophilisate for the preparation of solution for infusion reg. No.: P N008333 dated 01/27/10 - Indefinitely |
Release form, packaging and composition of the drug Soluvit N
Lyophilisate for the preparation of solution for infusion
in the form of a pressed yellow powder.
| 1 fl. | |
| sodium ascorbate | 113 mg, |
| which corresponds to the content of ascorbic acid (vit. C) | 100 mg |
| thiamine mononitrate | 3.1 mg, |
| which corresponds to the content of thiamine (vit. B1) | 2.5 mg |
| riboflavin sodium phosphate dihydrate | 4.9 mg, |
| which corresponds to the content of riboflavin (vit. B2) | 3.6 mg |
| sodium pantothenate | 16.5 mg, |
| which corresponds to the content of pantothenic acid (vit. B5) | 15 mg |
| pyridoxine hydrochloride | 4.9 mg, |
| which corresponds to the content of pyridoxine (vit. B6) | 4 mg |
| folic acid (vit. Bc) | 400 mcg |
| cyanocobalamin (vit. B12) | 5 mcg |
| Nicotinamide (Vit.PP) | 40 mg |
| biotin (Vit. H) | 60 mcg |
Excipients
: glycine - 300 mg, disodium edetate - 0.5 mg, methyl parahydroxybenzoate - 0.5 mg.
Colorless glass bottles (10) - contour cell packaging (1) - cardboard packs.
Soluvit N instructions for use
INSTRUCTIONS for using the drug Soluvit N
Marketing authorization holder: FRESENIUS KABI DEUTSCHLAND, GmbH (Germany)
Manufactured by: SINO-SWED Pharmaceutical Corporation (China) ATX Code: A11BA (Multivitamins)
Dosage form The drug is dispensed according to the prescription SOLUVIT N lyophilisate for preparation. r-ra d/inf.: fl. 10 pieces.
Release form, composition and packaging Lyophilisate for the preparation of a solution for infusion in the form of a pressed yellow powder. 1 fl. sodium ascorbate 113 mg, which corresponds to the content of ascorbic acid (vit. C) 100 mg thiamine mononitrate 3.1 mg, which corresponds to the content of thiamine (vit. B1) 2.5 mg riboflavin phosphate sodium dihydrate 4.9 mg, which corresponds to the content of riboflavin (vit. B2) 3.6 mg sodium pantothenate 16.5 mg, which corresponds to the content of pantothenic acid (vit. B5) 15 mg pyridoxine hydrochloride 4.9 mg, which corresponds to the content of pyridoxine (vit. B6) 4 mg folic acid (vit. Bc) 400 μg cyanocobalamin (vit. B12) 5 µg nicotinamide (Vit. PP) 40 mg biotin (Vit. H) 60 µg Excipients: glycine - 300 mg, disodium edetate - 0.5 mg, methyl parahydroxybenzoate - 0.5 mg.
Colorless glass bottles (10) - contour cell packaging (1) - cardboard packs.
Clinical and pharmacological group: Water-soluble vitamins for addition to solutions for parenteral nutrition
Pharmacotherapeutic group: Multivitamin
Indications : as part of total parenteral nutrition to meet the daily requirement for water-soluble vitamins in children and adults.
Dosage regimen Soluvit N must be dissolved under aseptic conditions immediately before use. Adults and children over 11 years old The contents of one bottle are aseptically dissolved in 10 ml of one of the following solutions: 1. Vitalipid N adult 2. Intralipid (10% or 20%) 3. Water for injection 4. Glucose solutions (5%, 10 %, 20%) The contents of the bottle, after dissolving in 10 ml of Vitalipid or Intralipid, are added under aseptic conditions to a fat emulsion (for example, Intralipid), or to a three-in-one parenteral nutrition system (Kabiven central and peripheral). The solution obtained by dissolving Soluvit N in 10 ml of water for injection or glucose solution is added under aseptic conditions to glucose solutions (5%, 10%, 20%), fat emulsion (for example, Intralipid), or to a parenteral nutrition system. three in one" (for example, Kabiven central or Kabiven peripheral). The contents of one bottle satisfy the daily need for vitamins. Adults and children over 11 years of age should receive 1 bottle per day. Children under 11 years old The contents of one bottle are dissolved under aseptic conditions by adding 10 ml of one of the following solutions: 1. Vitalipid N Children 2. Intralipid (10% or 20%) 3. Water for injection 4. Glucose solutions (5%, 10% , 20%) The contents of the bottle, after dissolving in 10 ml of Vitalipid or Intralipid, are added under aseptic conditions to a fat emulsion (Intralipid), or to a three-in-one parenteral nutrition system (Kabiven central and peripheral). The solution obtained by dissolving Soluvit N in 10 water for injection or glucose solution is added under aseptic conditions to glucose solutions (5%, 10%, 20%), fat emulsion (Intralipid), or to a three-in-one parenteral nutrition system "(Kabiven central and peripheral). Children weighing less than 10 kg: when adding 10 ml of a compatible solution to a bottle with Soluvit N, the daily requirement for water-soluble vitamins is provided by 1 ml of the resulting solution per 1 kg of body weight. Newborns and children weighing less than 10 kg should receive 1/10 of the contents of the bottle (1 ml of the resulting solution) per 1 kg of body weight per day.
Side effects During Soluvit N infusion, allergic reactions may occur in patients with a history of hypersensitivity to any component of the drug, for example, to thiamine or methyl parahydroxybenzoate. Contraindications for use: a history of hypersensitivity to any ingredient of the drug.
Special instructions Soluvit N must be dissolved immediately before use. The prepared solution of Soluvit N must be added under aseptic conditions to the infusion solution immediately before the start of the infusion and used within 24 hours. When diluting Soluvit N in aqueous solutions, the mixture must be protected from light. When diluting Soluvit N in Intralipid, this is not necessary, since the fat emulsion has light-protective properties.
Drug interactions Pyridoxine (vitamin B6) may reduce the effect of levodopa. Folic acid may reduce serum concentrations of phenytoin. In addition, the administration of folic acid in large doses may make the diagnosis of pernicious anemia difficult.
Conditions and periods of storage Store in a place protected from light, out of reach of children at a temperature not exceeding 25°C. Shelf life: 30 months.
Conditions for dispensing from pharmacies The drug is dispensed with a prescription.
Directions for use and doses
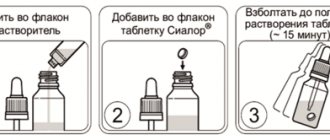
Soluvit N must be dissolved under aseptic conditions immediately before use.
Adults and children over 11 years old
The contents of one bottle are dissolved under aseptic conditions in 10 ml of one of the following solutions:
1. Vitalipid N adult
2. Intralipid (10% or 20%)
3. Water for injections
4. Glucose solutions (5%, 10%, 20%)
The contents of the bottle, after dissolving in 10 ml of Vitalipid or Intralipid, are added under aseptic conditions to a fat emulsion (for example, Intralipid), or to a three-in-one parenteral nutrition system (Kabiven central and peripheral). The solution obtained by dissolving Soluvit N in 10 ml of water for injection or glucose solution is added under aseptic conditions to glucose solutions (5%, 10%, 20%), fat emulsion (for example, Intralipid), or to a parenteral nutrition system. three in one" (for example, Kabiven central or Kabiven peripheral).
The contents of one bottle satisfy the daily need for vitamins. Adults and children over 11 years of age should receive 1 bottle per day.
Children under 11 years old
The contents of one bottle are dissolved under aseptic conditions by adding 10 ml of one of the following solutions:
1. Vitalipid N Children
2. Intralipid (10% or 20%)
3. Water for injections
4. Glucose solutions (5%, 10%, 20%)
The contents of the bottle, after dissolving in 10 ml of Vitalipid or Intralipid, are added under aseptic conditions to a fat emulsion (Intralipid), or to a three-in-one parenteral nutrition system (Kabiven central and peripheral).
The solution obtained by dissolving Soluvit N in 10 water for injection or glucose solution is added under aseptic conditions to glucose solutions (5%, 10%, 20%), fat emulsion (Intralipid), or to a three-in-one parenteral nutrition system "(Kabiven central and peripheral).
Children weighing less than 10 kg:
when adding 10 ml of a compatible solution to a bottle with Soluvit N, the daily requirement for water-soluble vitamins is provided by 1 ml of the resulting solution per 1 kg of body weight.
Newborns and children weighing less than 10 kg
should receive 1/10 of the contents of the bottle (1 ml of the resulting solution) per 1 kg of body weight per day.
Soluvit N
Release form, composition and packaging
Lyophilisate for the preparation of a solution for infusion in the form of a compressed yellow powder.
1 fl.
- sodium ascorbate 113 mg, which corresponds to the content of ascorbic acid (vit. C) 100 mg.
- thiamine mononitrate 3.1 mg, which corresponds to the content of thiamine (vit. B1) 2.5 mg.
- riboflavin sodium phosphate dihydrate 4.6 mg, which corresponds to the content of riboflavin (vit. B2) 3.6 mg.
- sodium pantothenate 16.5 mg, which corresponds to the content of pantothenic acid (vit. B5) 15 mg.
- pyridoxine hydrochloride 4.9 mg, which corresponds to the content of pyridoxine (vit. B6) 4 mg.
- folic acid (vit. Bc) 400 mcg.
- cyanocobalamin (vit. B12) 5 mcg.
- nicotinamide (Vit. PP) 40 mg.
- biotin (Vit. H) 60 mcg.
Excipients: glycine, disodium edetate, methyl parahydroxybenzoate.
Clinical and pharmacological group
Water-soluble vitamins for addition to parenteral nutrition solutions.
pharmachologic effect
Soluvit N is a lyophilized sterile powder containing water-soluble vitamins. It is used together with infusion solutions for intravenous administration and provides the daily need for water-soluble vitamins in children and adults.
Indications for use of the drug
- as a component of total parenteral nutrition to meet the daily requirement for water-soluble vitamins in children and adults.
Dosage regimen
Soluvit N must be dissolved under aseptic conditions immediately before use.
Adults and children over 11 years old
The contents of one bottle are dissolved under aseptic conditions in 10 ml of one of the following solutions:
- Vitalipid N adult
- Intralipid (10% or 20%)
- Water for injections
- Glucose solutions (5%, 10%, 20%)
The contents of the bottle, after dissolving in 10 ml of Vitalipid or Intralipid, are added under aseptic conditions to a fat emulsion (for example, Intralipid), or to a three-in-one parenteral nutrition system (Kabiven central and peripheral). The solution obtained by dissolving Soluvit N in 10 ml of water for injection or glucose solution is added under aseptic conditions to glucose solutions (5%, 10%, 20%), fat emulsion (for example, Intralipid), or to a parenteral nutrition system. three in one" (for example, Kabiven central or Kabiven peripheral).
The contents of one bottle satisfy the daily need for vitamins. Adults and children over 11 years of age should receive 1 bottle per day.
Children under 11 years old
The contents of one bottle are dissolved under aseptic conditions by adding 10 ml of one of the following solutions:
- Vitalipid N Children's
- Intralipid (10% or 20%)
- Water for injections
- Glucose solutions (5%, 10%, 20%)
The contents of the bottle, after dissolving in 10 ml of Vitalipid or Intralipid, are added under aseptic conditions to a fat emulsion (Intralipid), or to a three-in-one parenteral nutrition system (Kabiven central and peripheral).
The solution obtained by dissolving Soluvit N in 10 water for injection or glucose solution is added under aseptic conditions to glucose solutions (5%, 10%, 20%), fat emulsion (Intralipid), or to a three-in-one parenteral nutrition system "(Kabiven central and peripheral).
Children weighing less than 10 kg: when adding 10 ml of a compatible solution to a bottle with Soluvit N, the daily requirement for water-soluble vitamins is provided by 1 ml of the resulting solution per 1 kg of body weight. Newborns and children weighing less than 10 kg should receive 1/10 of the contents of the bottle (1 ml of the resulting solution) per 1 kg of body weight per day.
Side effect
During Soluvit N infusion, allergic reactions may occur in patients with a history of hypersensitivity to any component of the drug, for example, to thiamine or methyl parahydroxybenzoate.
Contraindications to the use of the drug
- history of hypersensitivity to any ingredient of the drug.
special instructions
Soluvit N must be dissolved immediately before use.
The prepared solution of Soluvit N should be added under aseptic conditions to the infusion solution immediately before the start of the infusion and used within 24 hours.
When diluting Soluvit N in aqueous solutions, the mixture must be protected from light. When diluting Soluvit N in Intralipid, this is not necessary, since the fat emulsion has light-protective properties.
Drug interactions
Pyridoxine (vitamin B6) may reduce the effect of levodopa.
Folic acid may reduce serum concentrations of phenytoin. In addition, the administration of folic acid in large doses may make the diagnosis of pernicious anemia difficult.
Conditions for dispensing from pharmacies
The drug is available with a prescription.
Storage conditions and periods
Store in a place protected from light, out of reach of children at a temperature not exceeding 25°C. Shelf life: 30 months.