Release form, packaging and composition of the drug Clinical-pharmacological group Pharmaco-therapeutic group Pharmacological action Indications for use Method of administration and doses Side effects Contraindications for use Use in children Special instructions Drug interactions
Registration Certificate Holder:
JODAS EXPOIM, Pvt. Ltd. (India)
ATX Code:
J01DH03
Active substance:
ertapenem
Dosage form:
Ertapenem J
| The drug is available with a prescription | Ertapenem J | Lyophilisate for the preparation of a solution for intravenous and intramuscular administration reg. No.: LP-005483 dated 04/22/19 - Valid |
Release form, packaging and composition of the drug Ertapenem J
Lyophilisate for preparing a solution for intravenous and intramuscular administration
in the form of a lyophilized powder or porous mass of white or yellowish-white color; the reconstituted solution should be clear, colorless or light yellow.
| 1 fl. | |
| ertapenem sodium | 1.046 g, |
| which corresponds to the content of ertapenem | 1 g |
Excipients
: sodium bicarbonate - 203 mg, sodium hydroxide qs to pH 6.5-8.5.
1 g - Colorless glass bottles with a capacity of 20 ml (1) - cardboard packs. 1 g - Colorless glass bottles with a capacity of 20 ml (5) - plastic trays (1) - cardboard packs.
pharmachologic effect
An antibiotic from the carbapenem group, it is 1-β methyl-carbapenem, a long-acting beta-lactam antibiotic for parenteral administration. Has a wide spectrum of antibacterial action.
The bactericidal activity of ertapenem is due to inhibition of cell wall synthesis and is mediated by its binding to penicillin binding proteins (PBPs). In Escherichia coli, it exhibits strong affinity for PBPs 1a, 1b, 2, 3, 4 and 5, with a preference for PBPs 2 and 3. Ertapenem has significant resistance to the action of most classes of β-lactamases (including penicillinases, cephalosporinases and β-lactamases extended spectrum, but not metallo-β-lactamase).
Active against
aerobic and facultative anaerobic gram-positive microorganisms:
Staphylococcus aureus (including penicillinase-producing strains), Streptococcus agalactiae, Streptococcus pneumoniae, Streptococcus pyogenes.
Active against aerobic and facultative anaerobic gram-negative microorganisms:
Escherichia coli, Haemophilus influenzae (including β-lactamase producing strains), Klebsiella pneumoniae, Moraxella catarrhalis, Proteus mirabilis.
Active
against anaerobic microorganisms:
Bacteroides fragilis and other Bacteroides spp., Clostridium spp. (except Clostridium difficile), Eubacterium spp., Peptostreptococcus spp., Porphyromonas asaccharolytica, Prevotella spp.
Methicillin-resistant staphylococci, as well as many strains of Enterococcus faecalis and most strains of Enterococcus faecium are resistant to ertapenem.
Also active against aerobic and facultative anaerobic gram-negative microorganisms:
Citrobacter freundii, Enterobacter aerogenes, Enterobacter cloacae, Escherichia coli (producing extended-spectrum β-lactamases), Haemophilus parainfluenzae, Klebsiella oxytoca, Klebsiella pneumoniae (producing extended-spectrum β-lactamases), Morganella morgani, Proteus vulgaris, Serratia marcescens.
Many strains of the microorganisms listed above that are multiresistant to other antibiotics, for example, penicillins, cephalosporins (including third generation) and aminoglycosides, are sensitive to ertapenem.
Active against anaerobic microorganisms
Fusobacterium spp.
Carbapenems occupy a special place among beta-lactam antibiotics due to their unique characteristics - a wide spectrum of antimicrobial activity and stability to the action of beta-lactamases, which determines the therapeutic value of this class of antimicrobial drugs (AMPs) in the era of increasing antibiotic resistance of pathogens of various infections [1]. Currently, three carbapenems are used in clinical practice: imipenem, meropenem and ertapenem. Another drug, doripenem, is in clinical trials.
According to the recently adopted classification, carbapenems are divided into two groups. The first includes ertapenem, a broad-spectrum AMP with limited activity against non-fermenting gram-negative bacteria, so its use is preferable for community-acquired infections. The second group includes imipenem, meropenem and doripenem, which are active against non-fermenting gram-negative bacteria and are used mainly for nosocomial infections [1]. It is also proposed to identify a third group, active against methicillin-resistant Staphylococcus aureus, for example, the drug CS-023, which is at the development stage [2].
Ertapenem (1 beta-methylcarbapenem), registered in Russia in 2002 under the trade name Invanz (Merck Sharp & Dohme Idea, Inc.), is a new long-acting parenteral carbapenem with a broad spectrum of antimicrobial activity against gram-positive and gram-negative aerobic and anaerobic flora.
Antimicrobial activity
Ertapenem is characterized by bactericidal activity due to disruption of cell wall formation when its molecule binds to penicillin-binding proteins (PBPs) [3, 4].
Gram-negative microorganisms
Ertapenem exhibits pronounced activity in vitro against microorganisms of the Enterobacteriaceae family (Escherichia coli, Klebsiella spp., Citrobacter spp., Enterobacter spp., Morganella morganii, Proteus spp., Serratia marcescens). Thus, the MIC90 * of ertapenem for most species of enterobacteria is ≤ 1 mg/l (Table 1). It is significant that ertapenem is active against Enterobacteriaceae producing extended spectrum beta-lactamases (ESBLs) and chromosomal beta-lactamases of the AmpC class, although the MIC90 values for these strains are higher [6, 7]. Ertapenem is also active against common respiratory pathogens - Haemophilus influenzae, producing and not producing beta-lactamases, and Moraxella catarrhalis with MIC90 values ≤ 0.25 mg/l [8, 9]. Unlike imipenem and meropenem, ertapenem does not have sufficient activity against non-fermenting gram-negative bacteria - Pseudomonas aeruginosa and Acinetobacter spp. [8–10].
Gram-positive microorganisms
Ertapenem is active against methicillin-sensitive Staphylococcus aureus, coagulase-negative staphylococci, Streptococcus pneumoniae, Streptococcus agalactiae, Streptococcus pyogenes, which are sensitive to it in 100% of cases (MIC90 ≤ 0.5 mg/l) (Table 1). Multidrug-resistant pneumococci, including strains resistant to tetracycline, clindamycin, or fluoroquinolones, are usually sensitive to ertapenem, but its activity against penicillin-resistant pneumococci is limited (MIC90 1-2 mg/L; 60-69.6% of susceptible isolates). Ertapenem has little activity against methicillin-resistant strains of S. aureus (MRSA), Enterococcus faecalis and Enterococcus faecium (MIC90 ≥ 16 mg/l) [8–10].
Anaerobes
Ertapenem exhibits pronounced in vitro activity against anaerobes: Bacteroides fragilis, Clostridium clostridioforme, Clostridium perfringens, Eubacterium lentum, Fusobacterium spp., Peptostreptococcus, Porphyromonas spp. and Prevotella spp. (MIC90 ≤ 4 mg/L, 97–100% susceptible isolates) (Table 1).
An important property of ertapenem is its resistance to hydrolysis by most beta-lactamases, including penicillinases, cephalosporinases and extended-spectrum beta-lactamases (ESBLs), with the exception of carbapenemases.
According to a clinical study conducted in the Russian Federation in patients with complicated intra-abdominal infections, all isolated strains of Enterobacteriaceae turned out to be sensitive to ertapenem, and among anaerobic bacteria the only strain (Bilophila wardsworthia) resistant to this AMP was found [12]. We emphasize that if the resistance of anaerobes to AMPs in the Russian Federation is not a problem, then among enterobacteria there is resistance to third-generation cephalosporins (9% of isolates), cefuroxime (29%), amoxicillin/clavulanate (40%), piperacillin (40%) and ampicillin (71%) due to the production of beta-lactamases, as well as to gentamicin (43%), ciprofloxacin (9%) and amikacin (3%) [12].
According to a multicenter study, the resistance of nosocomial strains of K. pneumoniae isolated in the ICU of various healthcare facilities in the Russian Federation was only 2.6% to ertapenem, while to ceftazidime - 57.1%, cefepime - 61.4%, piperacillin - 85 .9%, ciprofloxacin – 38.1%, gentamicin – 75.3%, amikacin – 31.4% [13].
Thus, in real clinical practice, ertapenem has clear advantages in activity against enterobacteria compared to AMPs of other classes.
Pharmacokinetics and dosage regimen
The bioavailability of ertapenem when administered intramuscularly is about 90% [13] (Table 2). The maximum concentration in plasma (C max) after intramuscular administration of 1 g is reached after approximately 2 hours (t max) [28]. The half-life (T½) is approximately 4 hours, allowing ertapenem to be administered once daily. Due to the presence of 1 beta-methyl group, ertapenem, unlike imipenem, is not destroyed by renal dehydropeptidase-1 [5]. There is no accumulation of the drug after repeated intravenous doses ranging from 0.5 to 2 g per day [28–30].
Ertapenem MIC90 values against many pathogens are lower than the mean plasma concentration over 24 hours and lower than that of non-plasma protein-bound ertapenem for ≥8 hours after a single 1 g intravenous dose.
Ertapenem actively binds to plasma proteins, primarily albumin. The steady-state volume of distribution of ertapenem (Vss) is approximately 5 L [29]. The drug penetrates well into tissues. Metabolized by hydrolysis to form an inactive derivative with an open
beta-lactam ring. It is excreted primarily by the kidneys by glomerular filtration and tubular secretion. Of the 80% of ertapenem determined in urine, 37.5% is excreted unchanged, and 36.7% is excreted as a metabolite [31].
The area under the pharmacokinetic curve (AUC) of ertapenem increases by 7% with mild renal impairment (creatinine clearance [CR] - 79 ml/min), by 53% with moderate renal impairment (CR 40 ml/min), by 158% in patients with severe renal failure (creatinine clearance 17 ml/min) and by 192% with end-stage renal disease (creatinine clearance < 10 ml/min).
For adults and children 13 years of age and older, ertapenem is recommended to be prescribed at a dose of 1 g once daily [13, 15]. For children from 3 months to 12 years, the drug is prescribed 15 mg/kg twice a day (no more than 1 g/day). According to the recommendations adopted in the Russian Federation for the prescription of ertapenem, in patients with severe/end-stage renal failure (creatinine clearance ≤ 30 ml/min/1.73 m2), as well as in patients receiving hemodialysis, the dose of the drug should be reduced to 500 mg/day [ 32]. Ertapenem is administered as a 30-minute intravenous infusion over 3–14 days. The duration of therapy may vary depending on the location and severity of the infection and the type of pathogen. In the Russian Federation, the drug is also approved for intramuscular administration (course duration is up to 7 days).
Possible drug interactions
Ertapenem does not inhibit the cytochrome P450 system and does not affect the pharmacokinetics of digoxin and vinblastine [13]. With the simultaneous administration of probenecid, the pharmacokinetic characteristics of ertapenem change: PFC increases by 25%, renal clearance decreases by 35%, and T½ is extended to 4.8 hours [13]. Ertapenem should not be mixed or administered with other drugs or with solutions containing dextrose.
Clinical use of ertapenem
The use of ertapenem is approved in the USA and Europe for the treatment of complicated intra-abdominal infections (CIAI), community-acquired pneumonia and acute infectious diseases of the pelvic organs; two more indications are additionally registered in the USA - treatment of complicated infections of the skin and soft tissues, including diabetic foot syndrome without signs of osteomyelitis, and complicated urinary tract infections (UTIs). In the Russian Federation, all of the listed indications for the use of ertapenem in adults and children from three months of age are registered. Recently, the indications for the use of ertapenem have been expanded. Now it can be used prophylactically during colorectal surgery.
Complicated skin and soft tissue infections
The efficacy of ertapenem was studied in comparison with piperacillin/tazobactam (PT) in 540 patients with complicated skin infections requiring parenteral therapy [16]. The main pathogen is methicillin-sensitive S. aureus. When prescribing ertapenem, the average duration of treatment was slightly shorter than when using PT (9.1 ± 3.1 and 9.8 ± 3.3 days, respectively). The effectiveness of ertapenem and PT turned out to be comparable: the clinical cure rate was 82.4 and 84.4%, respectively, pathogen eradication was 83% of cases in each group [16].
OIAI
The effectiveness of ertapenem in patients with AIAI has been studied in several comparative clinical studies. PT [17–19] or a combination of ceftriaxone and metronidazole [20] were used as comparison drugs. In most cases, OAI was caused by several microorganisms, with the most common pathogens being E. coli [17, 18, 20], B. fragilis [18, 20], other bacteroides [18] and Clostridium spp. [18].
In the treatment of OAI, the efficacy and safety of ertapenem were practically no different from those of comparator drugs. Thus, the clinical effectiveness when prescribing ertapenem was 82–94%, PT – 82–93% [17, 19], and bacteriological effectiveness – 87 and 81%, respectively [18]. Similar figures were obtained when comparing ertapenem with the ceftriaxone/metronidazole combination (84 and 85%, respectively) [20].
It is interesting that, despite the lack of sufficient in vitro activity of ertapenem against P. aeruginosa and enterococci, the clinical efficacy in patients with Pseudomonas aeruginosa infection with its use was 73% (PT - 89%), and with enterococcal infection - 77 (PT - 65 %) [18].
Complicated UTIs
The effectiveness of ertapenem was assessed in two clinical studies in patients with UTIs (mainly pyelonephritis) associated with foreign body obstruction or urinary tract abnormalities that impede normal bladder emptying [21, 22], and in a subsequent combined analysis of the results of these studies [ 23]. The comparison drug was ceftriaxone. The main causative agent of complicated UTIs was E. coli. According to the results of both studies, the effectiveness of ertapenem and ceftriaxone was proportional to the pathogen eradication rate - 86 and 85% [21], 92 and 93% [22], respectively, and in a combined analysis it was 89 and 91%, respectively [23].
Community-acquired pneumonia
Two clinical studies in patients with severe community-acquired pneumonia requiring parenteral antimicrobial agents compared the effectiveness of ertapenem (1 g/day intravenously) and ceftriaxone (1 g/day with the possibility of increasing the dose to 2 g/day if penicillin-resistant pneumococci are detected). The duration of therapy was 10–14 days [24, 25]. The main pathogens were S. pneumoniae, H. influenzae, M. catarrhalis and S. aureus. Clinical efficacy in the ertapenem treatment group was 92% (in both studies), ceftriaxone - 91 and 94%. The pathogen eradication rate was comparable between groups in both studies and was >90%.
Pelvic infections
In acute infectious diseases of the pelvic organs in women, the effectiveness of ertapenem was studied in comparison with PT [26]. AMPs were prescribed for 3–10 days. The clinical effectiveness of ertapenem was 94%, PT – 92%.
Diabetic foot syndrome
In October 2005, the US Food and Drug Administration (FDA) approved another indication for the use of ertapenem. The drug is approved for the treatment of moderate and severe complicated foot infections caused by S. aureus (methicillin-sensitive strains), S. agalactiae, S. pyogenes, E. coli, K. pneumoniae, P. mirabilis, B. fragilis, Peptostreptococcus spp., P. asaccharolytica and P. bivia, in patients with diabetes mellitus.
The basis for this decision was the results of a prospective, randomized, double-blind study (SIDESTEP), during which 586 patients with diabetic foot syndrome were treated with ertapenem 1 g/day or PT 3.375 g every six hours [27]. After five days of intravenous administration of these AMPs, patients were transferred to oral amoxicillin/clavulanate, the duration of which did not exceed 23 days. All patients underwent surgical treatment of the wound. When enterococci or methicillin-resistant strains of Staphylococcus aureus (MRSA) were isolated from clinical material or when there was a history of infection caused by MRSA, the main treatment regimen was supplemented with vancomycin.
At the time of discontinuation of intravenous therapy, the proportion of patients who experienced clinical recovery or improvement was 94% with ertapenem and 92% with PT. 10 days after completion of therapy, the results were also comparable: clinical recovery or improvement was noted in 87 and 83% of patients, respectively. Bacteriological effectiveness is similar in both groups (95 and 93%, respectively). The proportion of patients with clinical and bacteriological effectiveness of therapy was 85 and 82%, respectively [27].
Clinical research in pediatrics
The effectiveness of ertapenem was assessed in two randomized multicenter studies in children aged 3 months to 17 years with complicated bacterial infections [13]. The larger study included 404 patients with complicated UTIs, complicated skin and soft tissue infections, or community-acquired pneumonia. Ertapenem was administered intravenously at a dose of 15 mg/kg every 12 hours to patients aged 3 months to 12 years or 1 g once daily (13–17 years). Ceftriaxone was used as a comparison drug at a dose of 50 mg/kg/day intravenously in two (from 3 months to 12 years) or one (≥ 13 years) administration. In both groups, it is possible to transfer patients from parenteral therapy to oral amoxicillin/clavulanate. The total duration of antimicrobial therapy did not exceed 14 days. The microbiological effectiveness of therapy in patients with complicated UTIs was 87% in the ertapenem group and 90% in the ceftriaxone group. In patients with complicated skin and soft tissue infections, the clinical effectiveness of therapy was 96 and 100%, respectively, for community-acquired pneumonia - 96% in each group.
In another study of 112 patients with AIAI and pelvic infections, ertapenem was given at the same dosages as in the previous case, and a comparator drug was intravenous ticarcillin/clavulanate 50 mg/kg every 12 hours for body weight < 60 kg ; 3 g four to six times daily for weight ≥ 60 kg. The duration of therapy did not exceed 14 days. Clinical efficacy in patients with ACAI was 84% in the ertapenem group and 64% in the ticarcillin/clavulanate group; for infections of the pelvic organs, 100% clinical effectiveness was noted in all patients.
Key aspects of safety and tolerability
When used intravenously in patients with complicated bacterial infections, ertapenem is well tolerated. The most common adverse events (AEs) were diarrhea (5.5%), post-transfusion vascular complications (3.7%), nausea (3.1%), headache (2.2%), vaginitis (2.1%). ), phlebitis/thrombophlebitis (1.3%) and vomiting (1.1%) [7]. Convulsions during therapy with ertapenem were observed in 0.5% of patients, and during treatment with PT – in 0.3%. In terms of laboratory parameters, during treatment with ertapenem, increases in the activity of ALT (6.0% of patients), AST (5.2%), alkaline phosphatase (3.4%), platelet count (2.8%) and eosinophils ( 1.1%) [7].
The results of recent studies on the use of ertapenem and PT in patients with diabetic foot syndrome [27] and OAI [17] did not reveal statistically significant differences between the frequency of AEs (15 and 20% [27]; 13 and 12% [17]), serious AEs (0.3 and 0.3% [27]) or AEs that led to discontinuation of study drug therapy (1 and 2% [27]; 2 and 2% [17], respectively).
Results from a randomized, double-blind, multicenter study of intramuscular ertapenem (in 3.2 mL of 1% lidocaine) compared with ceftriaxone in patients with lower respiratory tract infections, skin infections, and UTIs showed that ertapenem was well tolerated by this route of administration. The most common post-injection reactions observed in patients are:
- pain on palpation (24 and 17%, respectively);
- pain at the injection site (17 and 23%);
- skin thickening (2 and 3%);
- hematoma (2 and 3%) [33].
The profile of AEs during ertapenem therapy in children is similar to that in adults. In children, when the drug was prescribed, the most common symptoms were diarrhea (6.5%), pain and redness at the injection site (5.5 and 2.6%), and vomiting (2.1%) [7]. Among the deviations in laboratory parameters in children, a decrease in the number of neutrophils (3.0%), an increase in the levels of ALT (2.2%) and AST (2.15%) were recorded [7].
Conclusion
Ertapenem is a new parenteral carbapenem with a broad spectrum of activity against gram-positive and gram-negative aerobic and anaerobic bacteria. Unlike third-generation cephalosporins, ertapenem is active against anaerobes and ESBL-producing microorganisms. T½, which is longer than other carbapenems and is stable to the action of dehydropeptidase-1, allows ertapenem to be used once a day in adults and children from three months of age as monotherapy in the treatment of community-acquired pneumonia, acute respiratory infections, pelvic infections, complicated infections of the skin, soft tissues, urinary tract.
Indications for use
Treatment of severe and moderate infectious and inflammatory diseases caused by sensitive strains of microorganisms (including for initial empirical antibacterial therapy until the pathogens are identified): infections of the abdominal cavity; infections of the skin and subcutaneous tissue, including infections of the lower extremities in diabetes mellitus (“diabetic” foot); community-acquired pneumonia; infections of the urinary system (including pyelonephritis); acute infections of the pelvic organs (including postpartum endomyometritis, septic abortion and postoperative gynecological infections); bacterial septicemia.
Directions for use and doses
The method of administration and dosage regimen of a particular drug depend on its release form and other factors. The optimal dosage regimen is determined by the doctor. The compliance of the dosage form of a particular drug with the indications for use and dosage regimen should be strictly observed.
Administered by intravenous infusion or intramuscular injection. When administered intravenously, the infusion duration should be 30 minutes. IM administration may be an alternative to IV infusion.
The average daily dose of the drug for adults is 1 g, the frequency of administration is 1 time/day.
The usual duration of therapy is from 3 to 14 days, depending on the severity of the disease and the type of microorganisms. If there are clinical indications, a transition to subsequent adequate oral antimicrobial therapy is acceptable.
In patients with CC>30 ml/min/1.73 m2, no dosage adjustment is required. In patients with severe renal impairment (creatinine clearance≤30 ml/min/1.73 m2), including those on hemodialysis, the recommended dose is 500 mg/day.
Patients on hemodialysis who received ertapenem at a dose of 500 mg/day in the next 6 hours before the hemodialysis session should receive an additional 150 mg of ertapenem after the session. If ertapenem is administered more than 6 hours before hemodialysis, no additional dose is required. There are currently no recommendations for patients undergoing peritoneal dialysis or hemofiltration.
Ertapenem J
Suction
Ertapenem dissolved in 1% lidocaine (without epinephrine) is well absorbed after intramuscular (IM) administration at the recommended dose of 1 g.
Bioavailability is approximately 92%. After intramuscular administration of 1 g per day, the maximum plasma concentration (Cmax) is reached after approximately 2 hours (Tmax).
Distribution
Ertapenem is highly bound to human plasma proteins (ertapenem protein binding decreases as plasma concentrations increase from approximately 95% at plasma concentrations <100 mcg/mL to 85% at plasma concentrations approximately 300 mcg/mL ).
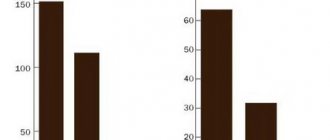
The mean plasma concentrations (µg/ml) of ertapenem following a single 30-minute intravenous (IV) infusion of 1 g or 2 g and a single 1 g intravenous dose in healthy volunteers are presented in Table 2.
table 2
| Plasma concentrations of ertapenem in adults after a single dose | |||||||||
| Dose - Route of administration | Average plasma concentrations (µg/ml) | ||||||||
| 0.5 h | 1 hour | 2 hours | 4 hours | 6 hours | 8 hours | 12 h | 18 h | 24 hours | |
| 1 g - i.v.* | 155 | 115 | 83 | 48 | 31 | 20 | 9 | 3 | 1 |
| 1 g - IM | 33 | 53 | 67 | 57 | 40 | 27 | 13 | 4 | 2 |
| 2 g - i.v.* | 283 | 202 | 145 | 86 | 58 | 36 | 16 | 5 | 2 |
| * IV infusion was carried out at a constant speed for 30 minutes. | |||||||||
The area under the plasma concentration-time curve (AUC) of ertapenem in adult patients increases almost directly proportional to dose over the dose range of 0.5 g to 2 g.
There is no accumulation of ertapenem in adult patients after repeated intravenous administration in the dose range from 0.5 g to 2 g per day or intramuscular administration of 1 g per day.
Mean plasma concentrations (μg/mL) of ertapenem in children are presented in Table 3.
Table 3
| Plasma concentrations of ertapenem in children after intravenous administration of a single dose* | ||||||||
| Age/dose | Average plasma concentrations (µg/ml) | |||||||
| 0.5 h | 1 hour | 2 hours | 4 hours | 6 hours | 8 hours | 12 h | 24 hours | |
| 3-23 months | ||||||||
| (15 mg/kg)** | 103,8 | 57,3 | 43,6 | 23,7 | 13,5 | 8,2 | 2,5 | — |
| (20 mg/kg)** | 126,8 | 87,6 | 58,7 | 28,4 | — | 12,0 | 3,4 | 0,4 |
| (40 mg/kg) *** | 199,1 | 144,1 | 95,7 | 58,0 | — | 20,2 | 7,7 | 0,6 |
| 2-12 years | ||||||||
| (15 mg/kg)** | 113,2 | 63,9 | 42,1 | 21,9 | 12,8 | 7,6 | 3,0 | — |
| (20 mg/kg)** | 147,6 | 97,6 | 63,2 | 34,5 | — | 12,3 | 4,9 | 0,5 |
| (40 mg/kg) *** | 241,7 | 152,7 | 96,3 | 55,6 | — | 18,8 | 7,2 | 0,6 |
| 13-17 years old | ||||||||
| (20 mg/kg)** | 170,4 | 98,3 | 67,8 | 40,4 | — | 16,0 | 7,0 | 1,1 |
| (1 g) | 155,9 | 110,9 | 74,8 | — | 24,0 | — | 6,2 | — |
| (40 mg/kg) *** | 255,0 | 188,7 | 127,9 | 76,2 | — | 31,0 | 15,3 | 2,1 |
| * — IV infusion was carried out at a constant speed for 30 minutes. ** - up to a maximum dose of 1 g/day *** - up to a maximum dose of 2 g/day | ||||||||
The volume of distribution of ertapenem in adult patients is about 8 liters (0.11 l/kg), in children aged 3 months to 13 years - 0.2 l/kg and about 0.16 l/kg in children aged 13-13 years. 17 years.
The concentration of ertapenem in breast milk of five lactating women, determined daily at random time points consecutively for 5 days after the last 1 g IV dose, was <0.38 mcg on the last day of treatment (5-14 days after delivery). /ml. By day 5 after cessation of treatment, ertapenem concentrations were below the detection limit in 4 women, and trace amounts (<0.13 μg/mL) were detected in 1 woman.
Ertapenem does not inhibit P-glycoprotein-mediated transport of digoxin and vinblastine, and is not itself a substrate for this transport (see section “Interaction with other drugs”).
Metabolism
After an intravenous infusion of 1 g of isotope-labeled ertapenem, the source of radioactivity in the plasma is mainly (94%) ertapenem. The main metabolite of ertapenem is an open-ring derivative formed by hydrolysis of the beta-lactam ring.
In vitro studies of human liver microsomes show that ertapenem does not inhibit metabolism mediated by the six major cytochrome P450 isoenzymes (CYP) - 1A2, 2C9, 2C19, 2D6, 2E1 and 3A4 (see section "Interaction with other drugs").
Removal
Ertapenem is excreted primarily by the kidneys. The average plasma half-life in healthy young volunteers and children aged 13-17 years is about 4 hours, in children from 3 months to 13 years - about 2.5 hours.
After IV administration of 1 g of isotope-labeled ertapenem to healthy young volunteers, approximately 80% of the drug is excreted by the kidneys and 10% by the intestines. Of the 80% of ertapenem detected in urine, about 38% is excreted as unchanged drug, and about 37% is excreted as a metabolite with an open β-lactam ring.
In healthy young volunteers who received a 1 g intravenous dose, the mean urinary concentration of ertapenem within 0-2 hours after administration of this dose exceeded 984 mcg/ml, and within 12-24 after administration of this dose exceeded 52 mcg/ml.
Features of pharmacokinetics in certain groups of patients
Floor. Plasma concentrations of ertapenem are comparable in men and women.
Elderly patients. Plasma concentrations of ertapenem following IV doses of 1 g and 2 g in older patients (>65 years) are slightly higher (approximately 39% and 22%, respectively) than in younger patients (<65 years). No dose adjustment is required for elderly patients.
Children. After intravenous administration of the drug at a dose of 1 g/day, the concentration of ertapenem in the blood plasma in children 13-17 years old and adult patients is comparable.
After administration of the drug at a dose of 20 mg/kg (up to a maximum dose of 1 g), the values of pharmacokinetic parameters in patients aged 13-17 years were generally comparable to those in healthy young volunteers. Three out of six patients aged 13-17 years received a dose of less than 1 g. To assess pharmacokinetic criteria in all patients in this group, the obtained indicators were calculated taking into account the fact that all patients received a dose of 1 g, assuming a linear relationship.
The comparison results show that the pharmacokinetic profile in patients 13-17 years of age receiving ertapenem at a dose of 1 g/day was comparable to that in adult patients. The ratios (patients 13–17 years of age/adult patients) for AUC, end-infusion concentration, and mid-dosing interval concentration were 0.99, 1.20, and 0.84, respectively.
Plasma concentrations at the midpoint of the dosing interval after a single IV administration of ertapenem at a dose of 15 mg/kg in patients aged 3 months to 13 years are comparable to those concentrations at the midpoint of the dosing interval after IV administration of the drug at a dose of 1 g/day. day in adults. Plasma clearance of ertapenem (ml/min/kg) in patients aged 3 months to 13 years was approximately 2 times greater than that in adult patients. At a dose of 15 mg/kg, AUC values in patients aged 3 months to 13 years were comparable to those in young healthy volunteers receiving ertapenem 1 g intravenously.
Patients with liver failure. The pharmacokinetics of ertapenem in patients with hepatic impairment have not been studied. Due to the low intensity of drug metabolism in the liver, it can be expected that disruption of its function should not affect the pharmacokinetics of ertapenem. No dosage adjustment is required in patients with liver failure.
Patients with renal failure. After a single intravenous administration of 1 g of ertapenem, the AUC in patients with mild renal failure (creatinine clearance Clcr 60-90 ml/min/1.73 m2) does not differ from that in healthy volunteers (aged 25 to 82 years).
In patients with moderate renal failure (CLcr 31-59 ml/min/1.73 m2), AUC is increased approximately 1.5 times compared to healthy volunteers.
In patients with severe renal failure (CLcr 5-30 ml/min/1.73 m2), AUC is increased approximately 2.6 times compared to healthy volunteers.
In patients with end-stage renal failure (Clcr <10 ml/min/1.73 m2), AUC is increased approximately 2.9-fold compared to healthy volunteers. After a single intravenous injection of a single dose of 1 g of ertapenem immediately before a hemodialysis session, about 30% of the administered dose is determined in the dialysate.
There are no data on the use of the drug in children with renal failure.
In patients with severe and end-stage renal failure, it is recommended to adjust the dosage regimen (see section "Dosage and Administration").
Side effect
From the side of the central nervous system:
often - headache; rarely - dizziness, drowsiness, insomnia, convulsions, confusion.
From the digestive system:
often - diarrhea, nausea, vomiting;
rarely - candidiasis of the oral mucosa, constipation, belching with sour contents, pseudomembranous colitis (often manifested by diarrhea) caused by uncontrolled proliferation of Clostridium difficile ,
dry mouth, dyspepsia, anorexia.
From the cardiovascular system:
rarely - decreased blood pressure.
From the respiratory system:
rarely - dyspnea.
Dermatological reactions:
often - rash; rarely - erythema, itching.
From the body as a whole:
rarely - abdominal pain, taste disturbance, weakness/fatigue, candidiasis, swelling, fever, chest pain.
Local reactions:
often - post-infusion phlebitis/thrombophlebitis.
From the genital organs
: vaginal itching.
From the laboratory parameters:
often - increased ALT, AST, alkaline phosphatase, increased platelet count; rarely - an increase in direct, indirect and total bilirubin, an increase in the number of eosinophils and monocytes, an increase in partial thromboplastin time, creatinine and blood glucose levels, a decrease in the number of segmented neutrophils and leukocytes, a decrease in hematocrit, hemoglobin and platelet count; bacteriuria, increased serum urea nitrogen levels, the number of epithelial cells in the urine, and the number of erythrocytes in the urine.
Other:
rarely - allergic reactions, general malaise, fungal infections.
special instructions
Serious (even fatal) anaphylactic reactions have been reported in patients treated with beta-lactam antibiotics. These reactions are more likely in individuals with a history of multivalent allergies (in particular, individuals with hypersensitivity to penicillin often develop severe hypersensitivity reactions when treated with other beta-lactam antibiotics). Before starting to use ertapenem, you should check for a history of indications of previous hypersensitivity reactions to other allergens (especially to penicillins, cephalosporins and other beta-lactam antibiotics). If an allergic reaction occurs, ertapenem should be discontinued immediately. When using ertapenem (like many antibacterial agents ) the development of pseudomembranous colitis (the main cause of which is a toxin produced by Clostridium difficile) is possible, which should be kept in mind when severe diarrhea occurs in patients receiving antibacterial therapy. When administered intramuscularly, avoid accidental penetration of ertapenem into a blood vessel. Use in pediatrics
Because The safety and effectiveness of ertapenem in pediatrics have not been studied, and its use in children and adolescents under 18 years of age is not recommended.
Note!
Description of the drug Invanz Liof. d/r-ra d/in. 1g fl. No. 1 on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.