- MagniuM
- Articles
- Magnesium tablets
|
|
Date of publication: 06/19/2019
Magnesium is a microelement without which the normal functioning of individual organs and the entire human body as a whole is impossible. This mineral takes part in a huge number of biochemical processes occurring in the human body. When there is a lack of an element, disturbances occur in the functioning of the nervous, digestive, and cardiovascular systems. Based on tests and the general clinical picture, the doctor prescribes the patient to take Mg in one form or another, as a rule, this is a mineral in tablets. Although today they can be replaced with other, safer means. For example, the functional drink “Magnium” enriched with this substance.
Which magnesium supplement should you choose?
October 25, 2022
20068
4.9
5
Content
- Why does the body need magnesium?
- What does magnesium do in the body?
- Symptoms of magnesium deficiency
- Top 5 magnesium preparations
- Magne Express
- Magne B6
- Magnerot
- Complivit Magnesium
- Panangin
- What foods are high in magnesium?
Magnesium is found in all tissues of the human body, because this essential element ensures the proper functioning of cells. Today we will tell you in what processes magnesium is involved, what are the symptoms of its deficiency and how to choose a good magnesium supplement.
Daily requirement
The average daily requirement for magnesium differs depending on age and gender:
| Population group | Daily value, mg | Upper permissible limit, mg |
| Infants (0-6 months) | 30 | — |
| 7-12 months | 75 | — |
| Children (1-3 years) | 80 | 145 |
| 4-8 years | 130 | 240 |
| 9-13 years | 240 | 590 |
| Girls (14-18 years old) | 360 | 710 |
| Boys (14-18 years old) | 410 | 760 |
| Men (19-30 years old) | 400 | 750 |
| Women (19-30 years old) | 310 | 660 |
| Men over 30 years old | 420 | 770 |
| Women over 30 years old | 320 | 670 |
| Pregnant and nursing | 450 | 750 |
Magnesium is not produced by the human body, but must be supplied to it through food. But it is quite difficult to cover the daily dose of this macronutrient through food alone. Taking this into account, one cannot do without the additional use of magnesium preparations. In this review, we have collected the best of them containing various salts of this macronutrient.
What does magnesium do in the body?
- Magnesium is necessary to provide the cell with energy.
- Without magnesium, B vitamins and vitamin C cannot be synthesized (that is, without magnesium, these vitamins do not enter the body).
- Magnesium interacts with calcium, strengthening bones, magnesium is necessary for muscle tone, it eliminates cramps and joint pain.
- Magnesium makes blood vessels strong, reduces blood pressure and regulates heart contractions (necessary for normal rhythm).
- Magnesium reacts with insulin, helping it penetrate cells, thereby regulating glucose levels.
- Magnesium helps skin heal after cuts and burns.
This is not a complete list of processes involving magnesium, but it is sufficient to understand how necessary the microelement is for our body.
Magnesium helps:
- in the prevention of headaches;
- cope with insomnia, stress and fatigue;
- relax the muscles;
- cope with depression faster;
- work of the musculoskeletal system;
- keep bones and teeth healthy. Read also How to strengthen the immune system: top 10 vitamins for schoolchildren The best vitamins for schoolchildren. Increases physical and mental endurance, strengthens the immune system, nervous system, bones and teeth.
Symptoms of magnesium deficiency
Here are at least 12 signs that you have a magnesium deficiency:
- Weakness. Since magnesium is involved in energy production, its deficiency immediately affects physical condition.
- Muscle twitching or spasms. Since magnesium helps muscles relax, its deficiency provokes involuntary contraction. If there were no magnesium in the body, our muscles would be constantly tense.
- Frequent headaches. Due to a lack of magnesium, serotonin levels decrease, hence vasospasm and a negative effect on the functioning of neurotransmitters. All this combined causes a headache.
- Insomnia. A lack of magnesium can cause sleep problems, especially if you are under stress. Stress hormones increase blood pressure and heart contractions.
- Irregular heartbeat. Due to a lack of magnesium, problems with heart rhythm are possible, and this is a fairly common occurrence. That is why doctors prescribe magnesium supplements for arrhythmia.
- Noise intolerance. A lack of magnesium affects the functioning of the nervous system, so sometimes a person reacts poorly to loud noise.
- Convulsions. Again, if there is little magnesium in the body, the nervous system suffers, this can even cause convulsions. You need to see a doctor urgently.
- Bones suffer from a lack of minerals. Magnesium is involved in the formation of bone tissue. If there is a lack of magnesium, calcium is poorly absorbed.
- Constipation. Without sufficient magnesium levels, intestinal function is disrupted. By the way, many laxatives contain magnesium.
- High blood pressure. Magnesium helps blood circulate properly, so its lack provokes surges in blood pressure.
- Diabetes mellitus type 2. Magnesium is involved in lowering blood sugar. If a person does not have enough magnesium in their diet, they may develop type 2 diabetes.
- Mood swings. If you feel a “swing” in your mood, be sure to donate blood to check your magnesium level.
In addition, with a lack of magnesium, loss of appetite and nausea, heartburn and acid reflux, brittle nails and skin problems are observed.
Remember that the symptoms listed may be a sign of other problems, so consulting a doctor is necessary. Do not self-medicate.
Symptoms of magnesium deficiency
Photos from open sources
Causes and manifestations of magnesium deficiency
A severe magnesium deficiency can be caused by one or several reasons:
- Reduced magnesium intake or increased magnesium costs. This is provoked by poorly balanced diets, chronic alcohol abuse or certain physiological conditions (growth period or age-related decline, stressful situations, childbearing and breastfeeding, sports).
- Redistribution of magnesium in the body. This can occur against the background of inflammatory or necrotic processes, with a reduced level of insulin in the blood. This can also be caused by heart surgery, chronic heart failure, and diabetes mellitus.
- Poor absorption of magnesium in the intestine or its rapid excretion through the gastrointestinal tract. The following pathological conditions lead to this: celiac disease, Crohn's disease, ulcerative colitis, Whipple's disease, removal of part of the intestine, frequent use of laxatives, malignant tumors.
- Impaired renal tubular absorption of magnesium. This is accompanied by acquired kidney pathologies (damage to nephron tubules and impairment of their function, kidney transplantation, syndrome of impairment of all renal functions).
- Endocrine diseases. In case of hyperfunction of the thyroid gland, Burnett's syndrome, and diabetes mellitus, magnesium deficiency is determined.
- Use of medications. First of all, magnesium metabolism disorders are caused by diuretic (diuretic) drugs.
- Magnesium deficiency is primarily indicated by disorders of the neuromuscular system and psyche. Muscle twitching, convulsions, increased reflexes, tremors, or muscle weakness are noted. Over time, the following may appear: laryngospasm, bronchospasm, vomiting, increased uterine tone. Possible addition of asthenia, mental disorders, manifested by anxiety, aggressiveness, fears.
Magne Express
This drug contains magnesium citrate and vitamin B6. This combination is very effective - these two substances enhance each other’s effects. "Magne Express" is prescribed for a slight lack of magnesium, as well as for the prevention of its deficiency. You can buy “Magnesium Express” in the form of a sachet (bags of granules for resorption). The drug is taken after meals (one sachet twice a day), there is no need to wash it down with water. The course of treatment is one month. The granules taste sweet and sour, with the aroma of tropical fruits. This magnesium citrate has high bioavailability, but is recommended to be taken as a dietary supplement. If there is a serious lack of magnesium, it is better to choose another drug (magnesium lactate), which will be prescribed by your doctor. Magne Express should not be taken during pregnancy, breastfeeding, kidney failure or while taking medications for Parkinson's disease. One of the disadvantages of this magnesium supplement is its high cost.
Magne Express
Pontroy Vitarmonil Industry, France
Magne Express is a dietary supplement that helps normalize the functioning of the nervous system and increases stress resistance.
from 66
4.5 2 reviews
667
- Like
- Write a review
Magnistad and Magnesol
Among the list of inexpensive and effective magnesium preparations, Magnistad should be mentioned (360 rubles/50 tablets). Contains Mg in the form of lactate, as well as pyridoxine. Therapeutic doses of substances can eliminate the deficiency that occurs due to poor nutrition, alcoholism, and dehydration.
Pyridoxine significantly improves the absorption of Mg in the intestine.
This form of the element is excreted by the kidneys, so it is contraindicated for people with chronic renal failure. Taking it on an empty stomach most often causes diarrhea, so take the drug with meals. Children over 6 years old and adults are recommended 6-8 tablets/day.
Another high-quality medicine with the element is Magnesol. It becomes a source of Mg, even with its acute deficiency. The medicine contains a citrate form of the substance, and the composition is enriched with riboflavin. The latter distinguishes Magnesol from most drugs that include pyridoxine. The price is 860 rubles/30 sachets, but the high cost is justified by the excellent quality. Magnesol is given to children from 11 years of age; a person needs 1-2 sachets per day, depending on the severity of the condition.
Magne B6
You can buy Magne B6 in two forms: tablets and oral solution. Let's consider the second option - Magne B6 in ampoules (but they are not injected, but drunk). The magnesium ampoule needs to be shaken, broken and poured into a cup of water. This magnesium is presented in the form of lactate. You need to take three to four of these ampoules a day. This drug is suitable not only for prevention, but also for quickly replenishing magnesium deficiency in the body. Magne B6 ampoules are a brownish liquid with a subtle caramel odor. This magnesium supplement is prescribed for gastrointestinal spasms, rapid heartbeat, pain and tingling in the muscles, irritability and mood swings. If a blood test reveals a lack of magnesium, Magne B6 in ampoules will quickly solve the problem. It is this form of the drug that is prescribed for laboratory-confirmed lack of magnesium in the blood. Magne B6 is often prescribed during pregnancy when there is malnutrition. Adults take up to four ampoules of magnesium per day (the dosage for children is calculated by the pediatrician, based on the baby’s weight). Once magnesium has returned to normal, the drug is well tolerated, acts quickly, and can be prescribed to small children. The relative disadvantages of Magne B6 are the price and the fact that the product is in ampoules. "Magne B6" is contraindicated in case of renal failure and while taking certain drugs for the treatment of parkinsonism.
Magne-B6
Sanofi-Winthrop Industries, Hungary
Magne B6 is based on a vital element present in all tissues and organs - magnesium.
This component takes an active part in all metabolic processes, including the transmission of impulses to nerve cells. The body primarily obtains magnesium from food. Its deficiency can occur in the event of an incorrect diet, for example, during diets, if the need for magnesium has sharply increased. The latter can happen during mental and physical stress, pregnancy, stress, and the use of diuretics. from 405
4.0 1 review
2282
- Like
- Write a review
Oral Bioavailability of Magnesium Oxide and Other Compounds (Review)
To date, there is no accurate data on the superiority of one or another magnesium preparation for oral use. Gastrointestinal absorption and other pharmacokinetic parameters of oral magnesium compounds are key to predicting the effectiveness of these drugs as a source of magnesium.
There is a widespread belief that organic magnesium compounds are better absorbed from the gastrointestinal tract than inorganic ones [1–3], however, the results of studies do not always confirm this point of view [4, 5], and the confirmatory ones are sometimes carried out not entirely correctly. For example, in a domestic study, the dynamics of magnesium concentration in blood plasma and erythrocytes was studied after a single administration of various dosage forms of magnesium preparations. The study had a cross-sectional design. 16 volunteers (11 men and 5 women) took part in it. Volunteers participated in the experiment repeatedly; between studies with the participation of the same volunteer, a gap of at least 15 days was maintained. Participants received Magne B6 8 tablets (Mg lactate dihydrate + pyridoxine, 384 mg based on pure Mg), Magne B6 3 ampoules (Mg lactate dihydrate + Mg pidolate + pyridoxine, 300 mg Mg). The comparison drug Berocca Plus was prescribed, according to the instructions, 1 tablet per day (Mg sulfate and carbonate, 100 mg Mg). Bioavailability was assessed as the area under the curve in plasma and erythrocytes. The differences in bioavailability between Magne B6 preparations and the control were statistically significant. The authors note a significantly higher bioavailability of organic magnesium in the form of both forms of Magne B6 compared to the reference drug, Berocca Plus. It is surprising that the authors compare the areas under the curve of drugs in which the magnesium content differs by at least 3 times [6].
It should be noted that, despite the active interest in the role of magnesium in metabolic processes, the effect of magnesium deficiency on the development of neurological and cardiovascular pathology, pathology of pregnancy, etc., studies studying the pharmacokinetics of oral magnesium preparations, including its oxide is very small [1].
In 1973, DA Cook published the results of a large experimental study of the pharmacokinetics of oral inorganic magnesium compounds in rats. After 5 days, the animals received a low magnesium diet for 14 weeks. magnesium in the form of magnesium oxide or chloride, or carbonate, or bicarbonate, or phosphate, or sulfate or silicate, or remained on a depleted diet. After this, the animals were killed and the levels of calcium and magnesium in the femurs, kidneys, urine, plasma, excrement were analyzed using the spectrophotometric method, then the absorption of magnesium was calculated: for carbonate it turned out to be 64.9%; for chloride – 61%, for oxide – 58%, for phosphate – 54.1%, for sulfate – 53.3%, for silicate – 54.2% [7].
In 1990, JS Lindberg et al. conducted an in vitro and in vivo study to compare the oral absorption of magnesium oxide and citrate in humans. The solubility of 25 mmol of both substances in 300 ml of hydrochloric acid solutions of different concentrations (0–24.2 mEq) and distilled water was compared. Magnesium oxide is practically insoluble in water and only 43% soluble in the most concentrated acid solution. Magnesium citrate in distilled water had a solubility of 55% and was more soluble than the oxide in acidic solutions. When the pH of the solutions was restored to 7 by titration with bicarbonate, neither the citrate nor the oxide recrystallized. Healthy volunteers received 25 mmol of either citrate or magnesium oxide orally. The level of salt absorption was assessed by changes in urinary excretion of magnesium. The increase in urinary magnesium levels was significantly higher in the group of volunteers receiving citrate [8].
In 1990, T. Bohmer et al. published the results of a study of magnesium excretion in healthy young female volunteers (students) within 24 hours after administration of magnesium in the form of hydroxide, citrate, magnesium lactate or placebo 3 times/day in a daily dose of 15–20.6 mmol. All drugs significantly increased the level of urinary excretion of magnesium, but there were no statistical differences in urinary excretion between participants taking different magnesium drugs. However, it should be noted that only 18 people took part in the study [9].
In 1994, SA Schuette et al. published data from a study of the intestinal absorption of magnesium oxide and chelated magnesium diglycinate, labeled with the 26Mg isotope, in patients (12 people) who had undergone ileal resection. The study had a double-blind, crossover design, with a dose of 100 mg. Magnesium oxide and diglycinate showed bioavailability of 22.8 and 23.5%, respectively, but there was a trend toward higher absorption of diglycinate in the 4 patients in whom the oxide was the worst absorbed. In addition, the peak plasma concentration of the isotope after taking diglycinate occurred earlier by 3.2 ± 1.3 hours [10]. AF Walker et al. (2003) studied the comparative pharmacokinetics of magnesium compounds (oxide, citrate and magnesium amino acid chelate (AAC)) in healthy volunteers in a double-blind, randomized, placebo-controlled study. Volunteers without signs of magnesium deficiency were randomized into 5 groups: receiving magnesium oxide 300 mg/day; magnesium citrate – 300 mg/day; Mg AAS, as well as 2 placebo groups: one took cellulose, the other took sorbitol. The drugs were taken for 60 days, patients were examined after the first day of administration and after 60 days of therapy. We studied magnesium levels in the blood (plasma and red blood cells), urine and saliva using atomic absorption spectrophotometry. Interestingly, in all groups, the initial average plasma magnesium levels were below normal. In blood plasma, citrate was the most effective. It significantly increased magnesium levels compared to AAS after 60 days of therapy, but there was no statistical difference between the results in the citrate and oxide groups. In this study, only the citrate group had significantly increased salivary magnesium levels. There were no differences between groups in erythrocyte magnesium levels. The researchers note that theoretically, magnesium oxide should cause a laxative effect, but the experiment participants who received it did not note such an effect of the drug [11].
C. Coudray et al. (2005) used a series of tests to investigate the intestinal absorption and urinary elimination, as well as the accumulation of various magnesium compounds in the body of male Wistar rats. The rats received magnesium oxide or magnesium chloride or sulfate or carbonate or acetate or pidolate or gluconate or citrate or lactate or aspartate. Before the study for 3 weeks. The rats received a diet with a reduced magnesium content (150 mg/kg). Then the rats in all groups received the same amount of magnesium in the form of its various compounds (550 mg/kg body weight). The experiment was continued for up to 6 weeks, and then the animals were sacrificed and the magnesium content in plasma, red blood cells and bones was measured using a precise method - inductively coupled plasma mass spectrometry. Plasma levels of magnesium, its content in red blood cells and bones after the use of different salts did not show significant differences, with a tendency to superiority when using magnesium gluconate. Finally, fecal and urinary excretion of magnesium were examined and intestinal magnesium absorption was calculated. The results demonstrate the lag of inorganic magnesium compounds (among them, magnesium oxide and chloride are better absorbed - 48.4% and 48.8%, respectively, sulfate is the worst - only 34.8%), and among organic salts the record holder is again gluconate - 56.8% .
The authors conclude that with a slight superiority of organic magnesium compounds (especially magnesium gluconate) and a slight lag in magnesium sulfate, all magnesium compounds are able to be absorbed and affect its levels in the blood and tissues [5].
In 2006, the results of a domestic study were published in the journal “Nutrition Issues” [Konyukhova O.S. et al.] pharmacokinetics of magnesium preparations and vitamins, conducted with the participation of 60 volunteers, of whom 15 received magnesium-containing preparations Magnerot (500 mg magnesium orotate; in terms of Mg2+ - 32.8 mg) once orally and another 15 people received Centrum (100 magnesium oxide; in terms of Mg – 60.3 mg). Based on the results of the study, the authors note that when taking the studied magnesium preparations in the body, an equal increase in the concentration of this element occurs in the body, but when taking magnesium oxide, it occurs at a later date [4].
The purpose of the study conducted at the Volgograd State Medical University [Spasov A.A. et al., 2010], there was a comparison of the rate of compensation of nutritional magnesium deficiency (Mg after the introduction of 8 inorganic and 12 organic magnesium salts), as well as an assessment of the ability of vitamin B6 to accelerate compensation of magnesium deficiency when combined with magnesium salts. To develop magnesium deficiency, 280 rats received a magnesium-deficient diet (magnesium content no more than 15 mg/kg) and distilled water for 7 weeks. A group of intact rats (12 animals) received a magnesium-balanced diet (Mg content – 500 mg per 1 kg of diet). Starting from the 49th day of the diet, the animals received magnesium salts (Mg chloride, Mg sulfate, Mg oxide, Mg nitrate, Mg thiosulfate, Mg hydrogen phosphate, Mg carbonate, Mg trisilicate, Mg L-, D- and DL-aspartate, Mg L- , and DL-pyroglutamate, Mg succinate, Mg glycinate, Mg orotate, Mg taurine, Mg lactate) or their combination with vitamin B6 at a dose of 50 mg of elemental magnesium and 5 mg of vitamin B6 per 1 kg of body weight. It was found that magnesium L-aspartate most effectively and quickly compensates for magnesium deficiency compared to all other magnesium salts. Among inorganic magnesium salts, the leader in the rate of compensation of magnesium deficiency was chloride, and the effectiveness of chloride immediately followed L-aspartate, ahead of other organic and inorganic salts. The effectiveness of the oxide was not inferior to either lactate or magnesium orotate [13].
The study of the bioavailability of magnesium preparations when taken orally continues. In Israel at the Medical Center. Chaim Shiba recently conducted a comprehensive comparative study of two compounds: magnesium oxide and magnesium citrate. Under observation were 41 healthy volunteers who did not have diagnosed heart disease. They were randomly distributed into two groups. For one month, the subjects in each group received one of two drugs available on the Israeli pharmaceutical market: magnesium citrate under the commercial name Magnesium Diasporal (295.8 mg of magnesium in one tablet) or magnesium oxide monohydrate under the commercial name Magnox 520. At the end of this month there was also a break in taking the drugs for 1 month, after which, already on the 3rd month. study, volunteers began taking magnesium supplements again, but each volunteer was already receiving a second drug for him: that is, those who first received magnesium citrate, this time took magnesium oxide, and vice versa. Before the start of each monthly drug intake and upon its completion, a study of magnesium concentrations in the blood serum and in the tissue cells of the volunteer’s body was carried out, platelet activity and electrolyte concentrations in the blood serum were studied. Volunteers were asked to fill out questionnaires regarding the quality of their daily lives. It was found that taking magnesium oxide significantly increased the concentration of magnesium in the body's cells and led to a decrease in the concentrations of low-density cholesterol and C-reactive protein. At the same time, taking magnesium citrate did not lead to such positive changes in laboratory parameters. The functional activity of platelets improved under the influence of both drugs [14].
Thus, the results of the few pharmacokinetic studies that determine the characteristics of the absorption of various magnesium salts from the gastrointestinal tract demonstrate a number of factors that impede the study of intestinal absorption of magnesium preparations.
Most studies of the pharmacokinetics of magnesium compounds have consisted of studying the level of urinary excretion of magnesium during the day and/or the concentration of magnesium ions in plasma/serum, which makes it possible only for an approximate assessment of intestinal absorption of magnesium. It should be remembered that plasma magnesium levels are subject to homeostatic control and magnesium can easily be lost from plasma to organs and tissues, and that plasma concentration is not an accurate indicator of intestinal magnesium absorption. Moreover, serum magnesium levels can remain within normal limits even when the total amount of magnesium in the body decreases by 80% due to the release of the trace element from the depot [15]. It can be said that to date there is no single generally accepted method for studying the effect of magnesium preparations on its content in the human body, and this makes it very difficult to study any pharmacokinetic parameters of these compounds. In addition, the rather limited number of participants in studies of the pharmacokinetics of magnesium preparations in humans and conflicting results are noteworthy.
Some authors consider it most appropriate to study the level of magnesium in erythrocytes and/or lymphocytes, as well as its concentration in saliva, but there is no consensus on this issue [5, 16, 17].
Based on the mechanisms of magnesium absorption in the intestine (passive diffusion along the electrochemical concentration gradient), it can be assumed that the lower the solubility, the better the absorption in the gastrointestinal tract. But the results of comparative studies indicate that the outsider in terms of bioavailability is not magnesium oxide (which is practically insoluble), but sulfate, which has good solubility (33.7 g in 100 g of water at 20°C) [5, 13].
Magnesium oxide, like other magnesium compounds, in experimental studies has proven the ability to successfully relieve deficiency of this element. Unfortunately, the above difficulties in assessing the bioavailability of magnesium compounds hinder the development of methodology for such studies. It is especially difficult to study the pharmacokinetics of magnesium compounds in humans. Modeling of deep magnesium deficiency, studying the level of magnesium in bones and other tissues, which have proven themselves well in experiments, are not applicable here. It must be remembered that the organization of objective studies of the pharmacokinetic parameters of magnesium compounds in humans should take into account the need to control the intake of magnesium from food, natural circadian (daily) changes in the level of endogenous magnesium in the blood, and determine the capacity of magnesium depots.
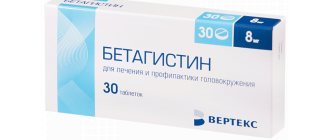
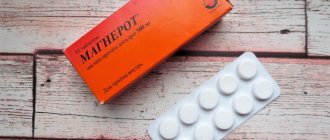
Magnerot
Magnerot is a magnesium orotate that is usually prescribed to patients with cardiac problems, although the drug can be taken for other symptoms of magnesium deficiency. “Magnerot” is indicated for people who have had a heart attack, with coronary heart disease, heart failure, arrhythmias, muscle pain, atherosclerosis, and lipid metabolism disorders. Magnerot is not cheap and has the same contraindications as previous drugs. In addition, Magnerot should not be taken if you have cirrhosis of the liver or urolithiasis. You need to take the drug 6 tablets per day (for a week). Then - one tablet three times a day.
Magnerot
Woerwag Pharma GmbH & Co. KG, Germany
Magnerot is a magnesium preparation containing magnesium orotate dihydrate.
Magnesium is a macroelement that takes part in energy, protein, lipid and carbohydrate metabolism, as well as the metabolism of nucleic acids. Magnesium inhibits neuromuscular transmission by regulating neuromuscular excitation. Magnesium is a natural calcium antagonist, takes part in the regulation of myocardial contractile function, and is necessary to maintain normal cardiomyocyte function. from 212
4.8 10 reviews
2089
- Like
- Write a review
Complivit Magnesium
This drug is a real vitamin-mineral complex, which, in addition to magnesium, contains B vitamins, vitamins A and E, ascorbic acid, calcium, copper, zinc. You need to take “Complivit Magnesium” one tablet daily. The cost of this magnesium preparation is low, and among the contraindications is only individual sensitivity to its components. A significant disadvantage is that Complivit Magnesium is not a medicine, but a dietary supplement, so it can only be prescribed to prevent magnesium deficiency.
Complivit Magnesium
JSC Pharmstandard-UfaVITA, Russia
A combined preparation containing a complex of vitamins and minerals, which are important factors in metabolic processes.
Used for: Prevention and replenishment of vitamin and mineral deficiencies. Increased physical and mental stress. The period of recovery after long-term and/or severe diseases, including infectious ones. In complex treatment when prescribing antibiotic therapy. from 120
5.0 1 review
835
- Like
- Write a review
Read also: Top 5 drugs for insomnia that are sold without a prescription The best over-the-counter drugs for the treatment of insomnia
Panangin
In addition to magnesium, this drug also contains potassium, so Panangin is usually prescribed for heart problems, in particular for rhythm disturbances. This remedy is indicated for elderly people with arterial hypertension. The fact is that while taking blood pressure medications with a diuretic effect, potassium is “washed out” of the body. The medicine should be taken only on the recommendation of a doctor. The maximum daily dose of Panangin is three tablets three times a day, and the course of treatment is a month. In addition to tablets, Panangin is also available in the form of injections for treatment in a hospital. This magnesium and potassium supplement is well tolerated and is quite affordable. Contraindications include renal failure, elevated levels of magnesium and potassium in the blood, as well as severe atrioventricular block. Pregnant women, especially in the first trimester, need to take Panangin with caution to prevent excess potassium.
Panangin
Gedeon Richter, Hungary
As part of complex therapy for heart failure, acute myocardial infarction, cardiac arrhythmias (mainly ventricular arrhythmias, as well as arrhythmias caused by an overdose of cardiac glycosides);
- to improve the tolerability of cardiac glycosides; — replenishment of potassium and magnesium deficiency when their content in the diet is reduced (for tablets). from 115
5.0 1 review
1121
- Like
- Write a review
Magnesium Rich Foods
Photos from open sources