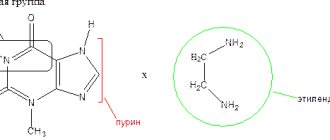
Compound
The film-coated tablet contains 150/300 mg ranitidine hydrochloride .
Excipients: silicon dioxide (colloid), MCC (type 12), copovidone, Mg stearate. Film shell components (white Opadry AMB OY-B28920): soy lecithin, talc, xanthan gum, titanium dioxide, polyvinyl alcohol .
Solution for injection (1 ml) contains 0.025 grams of ranitidine hydrochloride .
pharmachologic effect
Ranitidine Akos is an antiulcer drug, the active substance of which belongs to the group of histamine H2 receptor antagonists. The principle of action is based on blocking H2 receptors in parietal cells located in the gastric mucosa, as well as inhibiting the production of hydrochloric acid . Under the influence of the active substance, the volume of total secretion decreases, suppressing the activity of pepsin in gastric juice .
Thanks to the antisecretory effect of Ranitidine, it is possible to create favorable conditions for the healing of ulcerative lesions in the digestive tract (stomach, duodenum). The active substance is capable of exerting a protective effect by enhancing reparative processes, increasing the secretion of special mucous substances, and improving microcirculation.
Help with stomach diseases
Ranitidine: release form - tablets
Ranitidine is a drug used to treat stomach ulcers and chronic gastritis; it belongs to the group of histamine receptor blockers. The acidic environment of the stomach negatively affects the mucous membrane and destroys its walls.
If the mucous membrane is affected by ulcers, the acidic environment can cause gastric bleeding. Bleeding occurs due to the destruction of the walls of blood vessels in the tissues of the stomach. The action of an acidic environment on an ulcer can cause a perforated ulcer, which results in peritonitis and inflammatory processes in the abdominal cavity.
The stomach walls sometimes break down after taking medications that contain acid, such as Aspirin. Stress often negatively affects the health of the entire body, including the stomach, causing heartburn. While taking the drug, there is a gradual decrease in the concentration of gastric juice and a decrease in hydrochloric acid in its composition.
The amount of gastric juice produced gradually decreases. This allows you to get rid of the unpleasant burning sensation in the stomach and reduce pain. By acting on receptors, the drug stimulates the renewal of the mucous membrane and triggers the protective properties of cells by enhancing their microcirculation.
The drug is available in film-coated tablets and in the form of a solution.
Tablets can be prescribed without a doctor's prescription. The solution is used in hospitals to treat patients with stomach diseases, as well as to regulate digestive processes in the postoperative period.
Pharmacodynamics and pharmacokinetics
The active substance ranitidine is absorbed quite quickly from the lumen of the digestive tract. Food has no effect on the degree of absorption. Bioavailability reaches 50%. Peak concentrations are recorded within 2-3 hours after oral administration. 15% bound to plasma proteins. Partial metabolism takes place in the hepatic system with the formation of ranitidine S-oxide and desmethylranitidine .
The drug is characterized by a “first pass” effect through the hepatic system. The condition of the liver affects the extent and rate of elimination. After oral administration, the half-life is 2.5 hours, and with a creatinine clearance of 20-30 ml/min, this figure increases to 8-9 hours.
A small amount is excreted in the feces, the main part is excreted unchanged through the renal system. The active component does not pass the blood-brain barrier well, but penetrates the placenta well. Ranitidine is released during lactation.
Pharmacological properties of the drug Ranitidine
Ranitidine is an antisecretory agent, an H2-histamine receptor antagonist. The mechanism of action is due to competitive reversible inhibition of the action of histamine on H2 receptors of the membranes of parietal cells of the gastric mucosa. Suppresses basal and stimulated secretion of hydrochloric acid, caused, in particular, by baroreceptor irritation (gastric distension), food load, the action of hormones and biogenic stimulants (gastrin, histamine, acetylcholine, pentagastrin, caffeine). Reduces pepsin activity. Strengthens the protective mechanisms of the gastric mucosa and promotes the healing of its damage caused by acid exposure, by reducing gastric secretion and increasing the formation of gastric mucus, the content of glycoproteins in it, stimulating the secretion of bicarbonate ions by the gastric mucosa, endogenous synthesis of prostaglandins in it and regeneration processes. The duration of action for a single oral dose is 12 hours. When taken orally, the bioavailability of ranitidine is approximately 50%. The maximum concentration in blood plasma is achieved 2–3 hours after oral administration. With intramuscular administration, the maximum concentration in blood plasma is achieved in the first 15 minutes after administration. Ranitidine is excreted primarily in the urine. Partially metabolized in the liver. The half-life is 2–3 hours. About 93% of the dose administered intravenously and 60–70% of the dose taken orally are excreted in the urine, the rest in feces. Approximately 70% of ranitidine administered intravenously and about 35% taken orally are excreted unchanged in the urine. Penetrates through the placenta and into breast milk.
Indications for use of Ranitidine
Ranitidine tablets - what do they help with? The main area of application of the drug is gastroenterology .
Ranitidine Akos - what does it help with? The drug is prescribed for the treatment of various pathologies of the digestive system, and can also be used for prophylactic purposes.
Indications for use of Ranitidine Acri
- symptomatic ulcerative lesions of the digestive tract;
- peptic ulcer of the digestive system (stomach, duodenum);
- Zollinger-Ellison syndrome;
- prevention of aspiration of gastric juice during surgical interventions with the introduction of anesthesia;
- prevention of the development of “stress” ulcers;
- reflux esophagitis;
- erosive esophagitis;
- prevention of the development of ulcerative lesions of the gastrointestinal tract after surgical interventions;
- prevention of recurrent bleeding from the upper digestive tract.
Indications for use of Ranitidine Sopharma are similar.
Use for diseases of the esophagus
In diseases of the esophagus, in most cases, damage to its walls occurs with gastric acid due to insufficiency of the sphincter, which ensures compression of the lumen that closes the entrance to the stomach. Frequent releases of acidic contents damage the mucous membrane of the esophagus and purulent foci, as a result of inflammation, and ulcers form on its walls.
Such diseases include reflux esophagitis. Its symptoms include frequent and prolonged pain, heartburn, bad breath and belching. The disease is accompanied by neuroses and sleep disturbances. Taking Ranitidine relieves burning and pain, restores the mucous membrane by regulating the secretion of gastric juice and stimulating the work of the sphincter.
The drug is taken in the same dosage as for gastritis and ulcers - 300 mg; for severe pain, you can double the daily dose - 600 mg, in this case the medicine is taken 4 times.
Treatment of other diseases
Ranitidine is produced by different pharmaceutical companies
The drug can be used for erosive and ulcerative lesions of the intestines, benign neoplasms accompanied by heartburn. Tumors can affect the excess of the hormone gastrin in the body, which stimulates the production of gastric juice.
This can cause intestinal ulcers called Zollinger-Ellison syndrome. The drug restores the mucous membrane and slows down ulcerative manifestations. The drug is prescribed for this disease in a daily dosage of 450 mg, the dose is divided into three times.
Side effects
Hematopoietic system:
- leukopenia (with long-term therapy);
- thrombocytopenia.
The cardiovascular system:
- development of atrioventricular block (rarely, mainly with intravenous infusion).
Digestive tract:
- stool disorders ( constipation / diarrhea syndrome );
- hepatitis (extremely rare).
Side effects from the central nervous system:
- vertigo , dizziness ;
- fast fatiguability;
- blurred visual perception;
- headache;
- hallucinations (extremely rare);
- confusion (extremely rare).
Endocrine system, metabolism:
- increased prolactin ;
- increased creatinine ;
- amenorrhea;
- gynecomastia;
- decreased libido ;
- impotence.
Other reactions:
- recurrent mumps ;
- arterial hypotension;
- bronchospasm;
- arthralgia;
- hair loss;
- anaphylactic shock;
- angioedema;
- hives;
- various rashes on the skin;
- myalgia.
Side effects of the drug Ranitidine
Transient changes in liver function tests are possible; isolated cases of hepatitis (hepatocellular, cholestatic or mixed), usually reversible, accompanied or not accompanied by jaundice. Rare cases of acute pancreatitis have been described. From the hematopoietic system: rarely - reversible leukopenia, thrombocytopenia; isolated cases of agranulocytosis or pancytopenia, sometimes with bone marrow hypoplasia or aplasia. Allergic reactions: rarely - skin rash, urticaria, angioedema, anaphylactic shock, bronchospasm, arterial hypotension. From the cardiovascular system: rarely - bradycardia, AV block, asystole (with injection). From the central nervous system and sensory organs: rarely - headache, sometimes severe, dizziness; very rarely - reversible confusion and hallucinations, mainly in seriously ill and elderly patients; Some cases of the development of blurred visual perception, apparently caused by a violation of accommodation, have been described. From the endocrine status: very rarely - engorgement or discomfort in the mammary glands in men. Other side effects are rarely observed: arthralgia and myalgia.
Ranitidine tablets, instructions for use (Method and dosage)
The treatment regimen is selected individually. The tablets are intended to be taken orally.
Daily dosage is 300-450 mg (can be increased to 600-900 mg if necessary), divided into 2-3 doses. To prevent exacerbation of diseases of the digestive tract, the drug is prescribed at bedtime at a dose of 150 mg. The duration of therapy is determined by the dynamics of the disease.
For pathology of the renal system, the medication is prescribed twice a day at a dose of 75 mg. Instructions for use of Ranitidine Akos are similar. Your doctor will tell you how long you can take the pills (on average, the course of treatment is 2-4 weeks).
Use of the drug Ranitidine
Adults are administered intravenously slowly (at least 2 minutes) at a dose of 50 mg (in a volume of 20 ml), if necessary, repeated injections are carried out every 6–8 hours; IV drip at a rate of 25 mg/hour for 2 hours, if necessary, repeated administration after 6–8 hours. IM is prescribed at a dose of 50 mg (2 ml) every 6–8 hours. To prevent bleeding from stress ulcers in seriously ill patients or for the prevention of recurrent bleeding in patients with peptic ulcers of the stomach and duodenum, it is prescribed parenterally for the period when the patient cannot eat on his own. Patients with a continuing risk of bleeding may continue to take oral ranitidine 150 mg twice daily. For the prevention of bleeding from the upper gastrointestinal tract in seriously ill patients with stress ulcers, a slow IV administration of an initial dose of 50 mg followed by a continuous IV infusion of 0.125–0.25 mg/kg per hour is preferable. For patients at risk of developing acid aspiration, ranitidine is prescribed at a dose of 50 mg IM or IV slowly 45–60 minutes before general anesthesia. Orally, for active peptic ulcers of the stomach or duodenum, adults are prescribed 150 mg 2 times a day or 300 mg at night. In most cases, duodenal ulcers and benign gastric ulcers heal within 4 weeks. In some cases, scarring occurs later as treatment continues for the next 4 weeks. In the treatment of peptic ulcer of the duodenum, a dose of 300 mg 2 times a day is more effective than a dose of 150 mg 2 times a day and 300 mg at night. For anti-relapse treatment, 150 mg is prescribed at night; For patients who smoke, the dose is increased to 300 mg at night. When treating ulcers associated with taking NSAIDs, prescribe 150 mg 2 times a day or 300 mg at night for 8–12 weeks. For prophylactic purposes, when taking NSAIDs, 150 mg is prescribed 2 times a day. When treating postoperative stress ulcers, 150 mg is prescribed 2 times a day, usually for 4 weeks. Ulcers that do not heal within the specified period usually heal with continued treatment over the next 4 weeks. For reflux esophagitis, 150 mg 2 times a day or 300 mg at night is prescribed for 8 weeks; if necessary, the course of treatment can be extended to 12 weeks. For reflux esophagitis of II and III severity, the dose can be increased to 150 mg 4 times a day for 12 weeks. Long-term preventive therapy for reflux esophagitis - 150 mg 2 times a day. To eliminate pain from gastroesophageal reflux, 150 mg is prescribed 2 times a day for 2 weeks. If there is insufficient effectiveness, treatment can be continued at the same dose over the next 2 weeks. For Zollinger-Ellison syndrome, the initial dose is 150 mg 3 times a day; if necessary, the dose can be increased. Doses up to 6 g/day are usually well tolerated. For dyspepsia, 150 mg is prescribed 2 times a day for 6 weeks. If there is no positive effect from treatment, or if the condition worsens during treatment, a thorough examination of the patient should be carried out. To prevent bleeding from stress ulcers in seriously ill patients, as well as to prevent recurrent bleeding in patients with erosions, parenteral use of ranitidine can be replaced by oral administration at a dose of 150 mg 2 times a day, as soon as the patient is able to eat independently. To prevent the development of Mendelssohn's syndrome, a dose of 150 mg is prescribed orally 2 hours before anesthesia, and also (preferably) 150 mg the night before. During childbirth, women are prescribed 150 mg every 6 hours. For the treatment of peptic ulcers in children, the recommended dose of ranitidine is from 2 mg/kg to 4 mg/kg 2 times a day; the maximum daily dose is 300 mg.
Overdose
Main manifestations:
- skin rashes;
- confusion;
- headache;
- dizziness;
- increased drowsiness.
First aid consists of taking enterosorbents ( Polysorb , Smecta , Activated Carbon and others), calling an ambulance.
Interaction
There is a decrease in ranitidine absorption rates when treated with antacids . Elderly patients experience deterioration in attention and memory while taking anticholinergic drugs . It is assumed that medications that block histamine H2 receptors are able to suppress the ulcerogenic effect of drugs from the NSAID on the gastric mucosa. There is a decrease in the clearance of Warfarin during treatment with Ranitidine. Medical practice describes a case of bleeding and hypoprothrombinemia in a patient who was taking Warfarin .
There may be an undesirable increase in the absorption rates of ranitidine during simultaneous therapy with bismuth tripotassium dicitrate . Cases of hypoglycemia when taking Glibenclamide .
Ranitidine inhibits the absorption of Itraconazole and Ketoconazole . The half-life of Metoprolol and its AUC increase during treatment with Ranitidine. The absorption of the drug changes when taking high doses of Sucralfate (more than 2 g).
There is a slowdown in the excretion of Procainamide through the renal system, which leads to an increase in the concentration of the active substance in the blood. The absorption of Triazolam increases, which is associated with a change in the pH of gastric juice. The risk of toxicity increases with treatment with Phenytoin , which is explained by a significant increase in its concentration in the blood. There is an increase in the bioavailability of Furosemide with simultaneous therapy with Ranitidine.
In the medical literature there is a description of a case of the development of ventricular arrhythmia of the bigeminy in a patient who took Ranitidine and Quinidine . When treated with Cisapride, of cardiotoxicity increases . There is an increase in the level of Cyclosporine in the blood with parallel treatment with Ranitidine.
Drug interactions Ranitidine
With the simultaneous use of ranitidine with sucralfate in high doses (2 g), the absorption of ranitidine may be impaired, so the interval between their administration should be at least 2 hours. Ranitidine does not suppress the activity of the liver enzyme system associated with cytochrome P450, so it does not potentiate the effect of drugs, metabolized with the participation of this system (diazepam, lidocaine, phenytoin, propranolol, theophylline, warfarin). Increases the AUC and increases the concentration of metoprolol in the blood serum (by 80 and 50%, respectively), while the half-life of metoprolol increases. Reduces the absorption of itraconazole and ketoconazole.
List of pharmacies where you can buy Ranitidine:
- Moscow
- Saint Petersburg
special instructions
In case of severe pathology of the renal system, the medication is prescribed with caution. Before using the drug, it is necessary to exclude oncological diseases of the intestines , esophagus and stomach .
Long-term therapy of weakened patients who are in a state of stress can provoke the development of bacterial disease of the stomach, as well as the subsequent spread of the inflammatory process.
If the medication is abruptly discontinued, the risk of relapse of peptic ulcer increases. Preventive therapy is more effective with a course of taking the drug for 45 days in the fall and spring, compared to continuous use.
In patients suffering from various rhythm disorders, rapid intravenous administration of the solution can provoke bradycardia . For persons with of porphyria , Ranitidine is prescribed with caution due to the risk of developing an acute attack.
Distortion of laboratory test parameters (liver enzymes, creatinine, GGT) is allowed. The time interval between taking antacids and Ranitidine should be at least 1-2 hours due to the risk of changes in the absorption of the active substance. Clinical studies confirming the safety of the drug in pediatric practice are limited.
Interaction of ranitidine with other drugs and substances
Ranitidine may interact with other medications, which may impair treatment with this drug and any concomitant medications, if any.
The main drug interactions of ranitidine:
- antacids - the absorption of ranitidine worsens. The interval between doses must be at least 2 hours;
- antifungal drugs (itraconazole, ketoconazole) - ranitidine reduces the effectiveness of their therapeutic effect;
- metoprolol (a drug for high blood pressure) - ranitidine increases its concentration in the blood.
The interactions listed above for ranitidine are not exhaustive. To find out more, you should consult your doctor.
Ranitidine analogs
Level 4 ATC code matches:
Gastrosidine
Gistak
Acylok
Cimetidine
Zantac
Kvamatel
Famotidine
Structural analogues:
- Zantac;
- Gistak;
- Acylok;
- Ranisan.
Reviews of Ranitidine
The drug allows you to quickly relieve pain in the epigastric region due to ulcerative pathology of the digestive tract, gastropathy by reducing the acidity of gastric juice. Reviews about Ranitidine are mostly positive, because... The drug is well tolerated, causing virtually no negative symptoms if the dosage regimen is followed. Among the advantages are the low cost of the tablets and the quick effect in relieving heartburn.
The medication can be used in emergency cases in case of errors in the diet to prevent exacerbation of gastritis and peptic ulcers.
The downside is that it is impossible to use the medication during pregnancy and lactation.
Ranitidine 150 mg No. 20 tablets
Ranitidine 150 mg No. 20 tablets
Trade name RANITIDINE International nonproprietary name Ranitidine Dosage form coated tablets, 150 mg Composition One tablet contains the active substance - ranitidine hydrochloride 168 mg (equivalent to ranitidine 150 mg), excipients: potato starch, polyvinylpyrrolidone, modified corn starch, magnesium stearate. Opadry OY-C-7000 A shell composition: hydroxypropyl methylcellulose, titanium dioxide E171, ethylcellulose, diethyl phthalate). Description Tablets, coated in white with a grayish tint. Pharmacotherapeutic group Antiulcer drugs and drugs for the treatment of gastroesophageal reflux. H2-histamine receptor blockers. ATC code A02B A02 Pharmacological properties Pharmacokinetics Ranitidine is well absorbed from the gastrointestinal tract. Food intake does not have a significant effect on the absorption of the drug. Maximum plasma concentrations are achieved approximately 2 hours after oral administration. Bioavailability after oral administration is 50%. In small amounts, Ranitidine is metabolized in the liver. The plasma half-life is 2-3 hours. Approximately 30% of the dose taken is excreted unchanged in the urine within 24 hours. Renal clearance is approximately 410 ml/min, indicating active excretion by the renal tubules. The rate of elimination decreases if renal function is impaired. The main metabolite found in the urine, less than 4% of the dose taken, is N-oxide, other metabolites are S-oxide and demethylated ranitidine - 1% each. The rest of the administered dose of ranitidine is excreted in the feces. Ranitidine passes through the placental barrier and penetrates into mother's milk, where its concentration is higher than in the mother's blood plasma. It penetrates the blood-brain barrier to a small extent, so side effects from the central nervous system are rarely noted. Pharmacodynamics Ranitidine blocks H2-histamine receptors. The main effect is associated with a blocking effect on the receptors of the parietal cells of the stomach, as a result of which the secretion of the gastric glands, mainly the release of hydrochloric acid, decreases. The drug inhibits spontaneous gastric secretion; secretion caused by the action of histamine, pentagastrin, caffeine, as well as stretching of the stomach. Ranitidine does not have an antiandrogenic effect because it does not bind to androgen receptors. Ranitidine does not affect pepsin secretion. The drug does not particularly affect the secretion of internal factor stimulated by pentagastrin, and has little or no effect on gastrin levels. After taking a single oral dose of 150 mg, the concentration of the drug in the blood serum required to suppress the spontaneous secretion of hydrochloric acid by 50% is maintained for 12 hours. Indications for use peptic ulcer of the stomach and duodenum (including those associated with Helicobacter pylori) gastroesophageal reflux disease (GERD) treatment and prevention of postoperative, “stress” gastric ulcers prevention of bleeding in peptic ulcers of the stomach prevention and treatment of ulcers and erosions caused by the use of non-steroidal drugs anti-inflammatory drugs (NSAIDs) Zollinger-Ellison syndrome prevention of aspiration of gastric contents during childbirth and during general anesthesia Method of administration and dosage Ranitidine 150 mg is taken orally, regardless of meals, with water. Adults, elderly people and children over 16 years of age: Peptic ulcer of the stomach and duodenum: 150 mg 2 times a day (morning and evening) or 300 mg at bedtime. Course duration is from 4 to 8 weeks. When treating duodenal ulcers, the best results are achieved when using 300 mg 2 times a day for 4 weeks. Chronic episodic dyspepsia: 150 mg 2 times a day or 300 mg at bedtime for up to 6 weeks inclusive. For maintenance therapy, 150 mg is prescribed at night. If treatment does not give the expected effect, additional examination is necessary. Treatment and prevention of erosive and ulcerative lesions of the stomach and duodenum caused by the use of non-steroidal anti-inflammatory drugs (NSAIDs): to reduce the risk of erosion and ulcers, prescribe 150 mg 2 times a day or 300 mg before bedtime for 8 weeks. At the end of treatment, it is recommended to take 150 mg 2 times a day for prophylaxis; the duration of the course is prescribed individually. Erosive and ulcerative lesions of the stomach and duodenum caused by Helicobacter pylori infection: ranitidine 300 mg per day (in one or two doses), amoxicillin 750 mg 3 times a day and metronidazole 500 mg 3 times a day for 2 weeks. Treatment with ranitidine is continued for another 2 weeks. Maintenance therapy: 150 mg at night if there is a history of recurrent gastric ulcers. Gastroesophageal disease: stage I - ranitidine 150 mg per day for 6 weeks. Stage II - ranitidine 300 mg 2 times a day in combination therapy with proton pump inhibitors (PPIs) for up to 8 weeks inclusive. Grade III - ranitidine at a dose of 300 mg 2 times a day in combination therapy with PPIs and prokinetics for up to 8-12 weeks. Zollinger-Ellison syndrome: 150 mg 3 times daily. If necessary, the dose can be increased to 6 g per day inclusive. Treatment and prevention of postoperative, “stress” stomach ulcers, prevention of bleeding of peptic stomach ulcers: 150 mg 2 times a day. Prevention of aspiration of gastric contents during childbirth: 150 mg at the beginning of labor and after 6 hours. Patients at risk of aspiration syndrome: 150 mg 2 hours before anesthesia and preferably 150 mg the previous evening. In case of renal failure, it is not recommended to exceed the dose of 150 mg per day. Use is not recommended for children and adolescents under 16 years of age. If you miss the next dose of the drug, take the next dose at the usual time. Never take a double dose to replace a missed dose. Side effects Uncommon - headache, dizziness, increased fatigue, drowsiness, insomnia - constipation, diarrhea, dry mouth, nausea, vomiting, loss of appetite, abdominal pain, flatulence - skin rash Rare - hypersensitivity reactions (urticaria, fever, bronchospasm, chest pain), angioedema Very rare - hematopoietic disorders (agranulocytosis, leukopenia, thrombocytopenia, pancytopenia, sometimes with bone marrow hypoplasia or aplasia) - urticaria, bronchospasm, Quincke's edema, anaphylactic shock - reversible confusion, depression and hallucinations usually in elderly or seriously ill patients - transient disturbances in the form of spontaneous movements - blurred vision - tachycardia, bradycardia, AV block, extrasystole - vasculitis - pancreatitis - hepatitis (hepatocellular, cholestatic or mixed) with or without jaundice - erythema multiforme, transient alopecia - arthralgia, myalgia - interstitial nephritis - reversible functional disorders of the sexual sphere in men (decreased potency, gynecomastia) The drug is usually well tolerated. Side effects are reversible after reducing the dose or discontinuing the drug. Side effects usually disappear during treatment. Contraindications: hypersensitivity to ranitidine or the excipients of the drug; cirrhosis of the liver with a history of portosystemic encephalopathy; chronic renal failure (creatinine clearance; acute porphyria (history); pregnancy and lactation; childhood and adolescence up to 16 years of age; Drug interactions; absorption of ranitidine is reduced by antacids and sucralfate. Ranitidine should be apply 2-3 hours after using an antacid or sucralfate. There are isolated reports of increased plasma theophylline concentrations and side effects with the simultaneous use of ranitidine and theophylline, therefore the plasma theophylline concentration should be monitored and the theophylline dose adjusted if necessary. In very rare cases In cases where ranitidine interacts with an antidiabetic agent, the concentration of glucose in the blood plasma should be monitored and the dose of glipizide adjusted.Ranitidine increases the acidity of gastric juice, and therefore may affect the absorption of those drugs whose absorption depends on the degree of acidity of gastric juice (for example, ketoconazole, itraconazole) . With the simultaneous use of triazolam and ranitidine, the concentration of triazolam in the blood plasma may increase and its effect may be enhanced. Special instructions Before starting therapy, it is necessary to exclude the possibility of the presence of malignant neoplasms. If renal function is impaired (creatinine clearance). Sudden cessation of the drug may cause an exacerbation of peptic ulcer disease. Peculiarities of the drug's effect on the ability to drive a vehicle or potentially dangerous mechanisms. Caution should be exercised, since in rare cases the patient may experience side effects from the central nervous system. During the period treatment, you should refrain from smoking and drinking alcohol. Overdose Symptoms: increased effects listed in the “Side effects” section. Gait disturbances and hypotension are also noted. Treatment: forced induction of the gag reflex and/or gastric lavage, symptomatic therapy. For convulsions - diazepam in /c, for bradycardia - atropine, for ventricular arrhythmias - lidocaine. In severe cases - hemodialysis. Providing clinical monitoring of the patient. Release form and packaging 10 tablets are placed in a blister pack made of polyvinyl chloride film and printed aluminum foil. 2 packages together with instructions for medical use in the state and Russian languages are placed in a cardboard pack. Storage conditions Store in a dry place, protected from light, at a temperature not exceeding 25 °C. Keep out of the reach of children! Shelf life: 2 years Do not use after the expiration date indicated on the package. Conditions for dispensing from pharmacies By prescription
Ranitidine price, where to buy
The cost of tablets varies depending on the region of sale and pharmacy chain. The average price of Ranitidine in Russia is 30 rubles.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
LuxPharma* special offer
- Ranitidine TEVA (Zantac) tablet 300 mg 30 pcs
RUB 1,580 order - Zantac ampoules (Ranitidine in ampoules, Zantac) injection solution 25 mg/ml 2 ml No. 5
1970 rub. order
show more
Pharmacy24
- Ranitidine 150 mg N30 tablets PrAT "Tekhnolog", Uman, Cherkasy region, Ukraine
18 UAH. order - Ranitidine 150 mg N20 tablets PrAT "Technolog", Uman, Cherkasy region, Ukraine
12 UAH order
- Ranitidine-Darnitsa 0.15g No. 10 tablets PrAT” Pharmaceutical company “Darnitsa”, Ukraine
8 UAH order
- Ranitidine-Darnitsa 0.15g N20 tablets PrAT” Pharmaceutical company “Darnitsa”, Ukraine
15 UAH order
PaniPharmacy
- Ranitidine tablets Ranitidine tablets 150 mg No. 20 Ukraine, Tekhnolog ChAO
13 UAH order
- Ranitidine tablets Ranitidine-Zdorovye Forte tablets. p/o 300 mg No. 20 Ukraine, Health LLC
28 UAH order
- Ranitidine tablets Ranitidine-Zdorovye Forte tablets. p/o 300 mg No. 10 Ukraine, Health LLC
15 UAH order
- Ranitidine tablets Ranitidine tablets. p/o 0.15g No. 20 Ukraine, Darnitsa ChAO
19 UAH order
- Ranitidine tablets Ranitidine tablets. p/o 0.15g No. 10 Ukraine, Health LLC
8 UAH order
show more
Ranitidine solution for intravenous and intramuscular administration 25 mg/ml in 2 ml ampoules No. 10
Name
Ranitidine.
Release forms
Solution.
INN
Ranitidine.
FTG
H2-histamine receptor blocker.
Description
Transparent colorless or slightly yellowish liquid.
Compound
Each ampoule (2 ml of solution) contains: active ingredient: ranitidine (in the form of ranitidine hydrochloride) – 50 mg; excipients: sodium chloride, potassium dihydrogen phosphate, disodium phosphate dihydrate, water for injection.
Pharmacotherapeutic group
Drugs used to treat peptic ulcers and gastroesophageal reflux disease. Antagonist of H2-histamine receptors. ATX code - A02BA02.
Pharmacological properties
Pharmacodynamics II generation H2-histamine receptor blocker. The mechanism of action is associated with the blockade of H2-histamine receptors in the membranes of parietal cells of the gastric mucosa. Suppresses daytime and nighttime secretion of hydrochloric acid, as well as basal and stimulated, reduces the volume of gastric juice caused by gastric distension by food load, the action of hormones and biogenic stimulants (gastrin, histamine, acetylcholine, pentagastrin, caffeine). Reduces the amount of hydrochloric acid in gastric juice, practically without suppressing “liver” enzymes associated with cytochrome P450, and does not affect the concentration of gastrin in plasma or mucus production. Reduces pepsin activity. Does not affect the concentration of Ca2+ in the blood serum. A transient slight increase in the concentration of prolactin in the blood serum is possible after intravenous administration of ranitidine at a dose of 100 mg or more. It does not affect the release of pituitary hormones: gonadotropin, thyroid-stimulating hormone (TSH) and somatotropic hormone (STH). Does not affect the concentration of cortisol, aldosterone, androgens or estrogens, sperm motility, sperm quantity and composition, and does not have an antiandrogenic effect. May weaken the release of vasopressin. Strengthens the protective mechanisms of the gastric mucosa and promotes the healing of its damage associated with the effects of hydrochloric acid (including the cessation of gastrointestinal bleeding and scarring of stress ulcers), by increasing the formation of gastric mucus, the content of glycoproteins in it, and stimulating the secretion of bicarbonate by the mucous membrane stomach, endogenous synthesis of Pg in it and the rate of regeneration. Pharmacokinetics: Rapidly absorbed. The maximum concentration of the drug in blood plasma (Cmax) is 300–500 ng/ml (after intramuscular administration); TCmax 15–30 min. Communication with plasma proteins – 15%. Poorly penetrates the blood-brain barrier (BBB); penetrates the placental barrier and into breast milk (the concentration in breast milk of a woman during lactation is higher than in plasma). Slightly metabolized in the liver to form desmethylranitidine, ranitidine S-oxide and ranitidine N-oxide and a furoic acid analogue. Has a “first pass” effect through the liver. The rate and degree of elimination depend little on the condition of the liver. T1/2 after intravenous administration - 1.9 hours. Excreted by the kidneys: with intravenous administration - 93% (mainly unchanged 70%) and through the intestines.
Indications for use
Ranitidine is indicated for the treatment of duodenal ulcers, benign gastric ulcers, postoperative ulcers, reflux esophagitis, Zollinger-Ellison syndrome and the prevention of the following conditions in which it is necessary to reduce gastric secretion and reduce the formation of hydrochloric acid: prevention of gastrointestinal bleeding in stress ulcers in severe patients, prevention of recurrent bleeding in patients with bleeding gastric ulcers, and before general anesthesia in patients at risk of acid aspiration (Mendelssohn syndrome), especially during childbirth. Ranitidine injections are indicated for short-term use in hospitalized patients with pathological hypersecretory conditions who are unable to take oral medications.
Contraindications
- acute porphyria (including history); - pregnancy; - lactation period (breastfeeding); - children under 12 years of age; - hypersensitivity to ranitidine and other components of the drug.
Carefully
The drug should be prescribed with caution in case of renal and liver failure, cirrhosis of the liver with a history of portosystemic encephalopathy.
Directions for use and doses
Parenterally (intravenously, intramuscularly). Intravenously slowly (over 5 minutes) 50 mg, diluted with 0.9% sodium chloride solution or 5% dextrose solution to 20 ml; if necessary, repeat administrations are carried out every 6-8 hours. Intravenous drip, at a rate of 25 mg/hour for 2 hours; if necessary, repeat administration after 6-8 hours. Intramuscularly - 50 mg 3-4 times a day. To prevent bleeding from the upper gastrointestinal tract in patients with stress ulcers, slow intravenous administration is preferable at an initial dose of 50 mg, followed by continuous intravenous infusion at a rate of 0.125-0.25 mg/kg/h. The administration is carried out until the patient is able to eat on his own. To prevent the development of Mendelssohn's syndrome - intramuscularly or slowly intravenously, 50 mg 45-60 minutes before general anesthesia. In patients with renal failure (creatinine clearance less than 50 ml/min), accumulation of ranitidine may occur with increased plasma concentrations. Accordingly, in such patients ranitidine is prescribed at a dose of 25 mg. For patients on hemodialysis, the next dose is prescribed immediately after the end of hemodialysis.
Side effect
Below are the adverse events depending on the frequency of their occurrence in the following groups: very often (≥ 1/10), often (from ≥ 1/100 to
Interaction with other drugs
When taking ranitidine and drugs that depress bone marrow simultaneously, the risk of developing neutropenia increases. When used together with coumarin anticoagulants (for example, warfarin), prothrombin time may change. Due to the narrow therapeutic index, careful monitoring of prothrombin time is necessary. High doses of ranitidine (eg, used in the treatment of Zollinger-Ellison syndrome) may decrease the elimination of procainamide and N-acetylprocainamide, resulting in increased plasma levels of these drugs. Ranitidine inhibits the metabolism of phenazone, aminophenazone, hexobarbital, indirect anticoagulants, glipizide, buformin, and calcium antagonists. Due to an increase in the pH of the gastric contents, when administered concomitantly with ranitidine, the absorption of itraconazole and ketoconazole may be reduced. When taken against the background of ranitidine, the AUC and concentration of metoprolol in the blood serum increases (by 80% and 50%, respectively), while T1/2 of metoprolol increases from 4.4 to 6.5 hours. There is no interaction of ranitidine with metronidazole and amoxicillin.
Pharmaceutical interactions
Ranitidine solution is compatible with the following infusion solutions: 0.9% sodium chloride solution, 5% dextrose solution, 0.18% sodium chloride solution and 4% dextrose solution, 4.2% sodium bicarbonate solution, Hartmann's solution.
Precautionary measures
Evaluation for the presence of malignancy. Before starting treatment, the presence of malignant neoplasms in the stomach and duodenum should be excluded (may mask the symptoms of stomach cancer). Use for renal dysfunction. In patients with severe renal failure (creatinine clearance less than 50 ml/min), accumulation and increased plasma concentrations of ranitidine are observed. The recommended dose is 150 mg 1 time per day. For patients undergoing long-term ambulatory peritoneal dialysis or long-term hemodialysis, the drug is prescribed at a dose of 150 mg immediately after the end of the dialysis session. In patients with heart disease, rapid intravenous administration and use in high doses increases the risk of cardiotoxic effects (bradycardia). The use of ranitidine in doses higher than recommended may lead to increased creatinine concentrations, glutamate transpeptidase activity and hepatic transaminases in the serum. Ranitidine can provoke attacks of acute porphyria, so its use should be avoided in patients with a history of attacks of porphyria. Elderly patients, patients with chronic lung disease, diabetes mellitus, or those who are immunocompromised have an increased risk of developing community-acquired pneumonia (1.82 times (95% CI: 1.26–2.64)) compared with patients who did not receive ranitidine ). In elderly patients and patients with severe health problems, the use of ranitidine may lead to confusion, depression and hallucinations. With long-term treatment of weakened patients under stress, bacterial damage to the stomach is possible with subsequent spread of infection. Ranitidine should not be abruptly discontinued; there is a risk of rebound syndrome. Symptoms of duodenal ulcer may disappear within 1-2 weeks, but therapy should be continued until scarring is confirmed by endoscopic or x-ray examination. Ranitidine should be taken 2 hours after the use of itraconazole or ketoconazole to avoid a significant decrease in their absorption. The use of ranitidine may cause a false-positive reaction when testing for the presence of protein in the urine. It counteracts the effect of pentagastrin and histamine on the acid-forming function of the stomach and mast cells, therefore, it is not recommended to use it within 24 hours preceding the test, as well as before conducting diagnostic skin tests with histamine to detect an immediate allergic skin reaction. Smoking reduces the effectiveness of the drug. During treatment, you should avoid consuming foods, drinks and other medications that may irritate the gastric mucosa.
Use during pregnancy and breastfeeding
Ranitidine crosses the placenta and is excreted in breast milk (concentrations in breast milk are higher than in plasma). The use of a drug during pregnancy is possible only if the expected benefit to the mother outweighs the potential risk to the fetus. If it is necessary to prescribe a drug during lactation, the issue of stopping breastfeeding should be decided.
Use in pediatrics
The safety and effectiveness of ranitidine in children under 12 years of age have not been established.
Use for liver dysfunction
The drug should be prescribed with caution in case of liver failure, cirrhosis of the liver with a history of portosystemic encephalopathy.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Overdose
Symptoms: convulsions, bradycardia, ventricular arrhythmias. Treatment: symptomatic. For the development of seizures, intravenous diazepam; for bradycardia, atropine; and for ventricular arrhythmias, lidocaine. Hemodialysis is effective.
Package
2 ml in glass ampoules. 10 ampoules along with the package insert are placed in a cardboard box (No. 10). 10 ampoules, together with a leaflet, are placed in a cardboard pack with a cardboard insert for fixing the ampoules (No. 10).
Storage conditions
In a place protected from light, at a temperature not exceeding 25°C. Keep out of the reach of children.
Best before date
2 years. The medicine cannot be used after the expiration date.
Conditions for dispensing from pharmacies
On prescription.
Buy Ranitidine solution for IV and IM administration 25 mg/ml in ampoules 2 ml No. 10 in the pharmacy
Price for Ranitidine solution for IV and IM administration 25 mg/ml in 2 ml ampoules No. 10
Instructions for use for Ranitidine solution for IV and IM administration 25 mg/ml in 2 ml ampoules No. 10