Compound
Active ingredient:
INN - tiotropium bromide 22.5 mcg (respectively 18 mcg tiotropium ) in one capsule and 3.1235 mcg tiotropium bromide (respectively 2.5 mcg tiotropium ) in one dose of inhalation solution.
In addition to the active ingredient, the capsules contain lactose monohydrate.
In addition to the active ingredient, the inhalation solution includes: benzalkonium chloride, hydrochloric acid, disodium edetate, water.
Pharmacodynamics and pharmacokinetics
Tiotropium is a long-acting antimuscarinic drug ( anticholinergic drug ) and exhibits equal affinity for different muscarinic type receptors from M1 to M5. smooth muscles relax , which in turn leads to the bronchodilator effect of the drug, which, depending on the dose taken, lasts for 24 hours or even longer. With the inhalation method of administration, the effect of tiotropium is selective local in nature, without leading, in therapeutic doses, to systemic anticholinergic negative effects. Thus, bronchodilation is manifested not by a systemic, but by a local effect of the drug.
30 minutes after inhalation of 1 dose, a significant increase in lung function occurs, which continues throughout the day. Pharmacodynamic equilibrium was observed within 7 days of administration, and a pronounced bronchodilator effect was achieved on the 3rd day. There were no manifestations of tolerance to Spiriva when assessing its bronchodilator effect over a 12-month period. Taking the drug reduces the number of exacerbations of COPD compared to placebo, lengthens the time to the first exacerbation, has a positive effect on the quality of life throughout the entire period of use, reduces the number of forced hospitalizations associated with exacerbation of COPD , and also delays the time of first hospitalization .
The absolute bioavailability of tiotropium when taken in the form of inhalations is 19.5%, which guarantees its high content in the lungs with an insignificant effect on the patient’s body as a whole. from the gastrointestinal tract , and therefore its absorption does not depend on food intake. In patients with COPD, Cmax is reached 5 minutes after inhalation and is 17–19 pg/ml, with a steady-state plasma concentration of 3–4 pg/ml. It is 72% bound to plasma proteins, with a volume of distribution of 32 l/kg. Does not have the ability to pass the BBB .
Subject to minor biotransformation . The degradation pathway is non-enzymatic and results in the formation of dithienylglycolic acid and the alcohol N-methylscopine , which do not bind to muscarinic receptors .
Terminal T1/2 after inhalation - 5–6 days.
14% of the dose is excreted in the urine by the kidneys, the remaining in feces.
Pharmacological properties of the drug Spiriva
Pharmacodynamics. Tiotropium bromide is a specific anticholinergic agent with a long-lasting effect, which has an affinity for the M1-M5 subtypes of muscarinic receptors. By competitively and reversibly blocking receptors of the M3 subtype in the respiratory tract, it leads to relaxation of bronchial smooth muscles. in vitro and in vivo studies, the bronchodilator effect was dose-dependent and lasted more than 24 hours. This duration of effect is likely due to the very slow release of the drug from M3 receptors, which determines a long half-life and significantly exceeds that of ipratropium bromide. Being a quaternary ammonium compound, tiotropium bromide has a predominantly local effect when administered inhaled; its systemic anticholinergic effects are weakly expressed. Dissociation from binding to M2 receptors is faster than from binding to M3 receptors in vitro . M3 is a more acceptable (kinetically controlled) receptor subtype. The drug's high efficacy and slow dissociation from receptors have been clinically correlated with significantly more pronounced and prolonged bronchodilation in patients with COPD. Bronchodilation following inhaled tiotropium is primarily a local effect on the airways rather than a systemic one. The use of Spiriva once a day was accompanied by a significant increase in lung function (increase in forced expiratory volume in the first second and forced vital capacity) within 30 minutes after inhalation, the duration of the effect was 24 hours. A stable pharmacotherapeutic effect was achieved within 1 week from the start of treatment . When used daily, Spiriva significantly increases morning and evening maximum expiratory flow. Improvement in lung function during treatment lasts for a long period; no signs of developing tolerance to the drug were noted. Bronchodilation lasts over the 24-hour dosing interval compared to placebo. The mode of drug administration (morning or evening) was not taken into account. In long-term studies (over the course of a year) it was established:
- Spiriva significantly reduces shortness of breath; the improvement was maintained throughout the entire treatment period;
- significantly reduces the number of exacerbations of COPD and stops the occurrence of the first exacerbation;
- significantly improves quality of life; improvement was noted throughout the entire treatment period;
- significantly reduces the number of hospitalized patients with exacerbation of COPD, while the first hospitalization is required later.
2 studies found that taking Spiriva significantly increased tolerance to physical activity limited by symptoms of the disease - by 19.7 and 28.3%, respectively. In a study of the use of 18 and 54 mcg (3 times a day, 18 mcg) of Spiriva for 12 days, there was no prolongation of the QT according to ECG indicators. Pharmacokinetics. Tiotropium bromide is a quaternary ammonium compound that is poorly soluble in water, so it is used for inhalation in powder form. As a rule, most of the dose inhaled during inhalation is deposited on the walls of the pharynx and then swallowed, while a smaller part is deposited on the walls of the bronchi. After inhalation of the dry powder in healthy young volunteers, the absolute bioavailability was 19.5%, which is a sign of high bioavailability of the fraction reaching the lungs. Taking into account the chemical structure of the active substance (quaternary ammonium compound), it is assumed that tiotropium bromide is absorbed only to a small extent in the digestive tract. For the same reason, simultaneous food intake does not affect its absorption. The absolute bioavailability of tiotropium bromide in the form of oral solution is 2–3%. The maximum concentration of tiotropium bromide in blood plasma is observed 5 minutes after inhalation. 72% of the drug binds to blood plasma proteins. Volume of distribution - 32 l/kg. At steady state, the maximum level of tiotropium bromide in the blood plasma in patients with COPD was 17–19 pg/ml when determined 5 minutes after inhalation at a dose of 18 mcg and then rapidly decreased in a stepwise manner. The local concentration in the lungs is unknown, but based on the route of administration, high concentrations in the lungs are acceptable. Tiotropium bromide does not penetrate the BBB to a significant extent. The degree of biotransformation is insignificant. Tiotropium ester breaks down nonenzymatically to form the alcoholic N-methylscopine and dithienylglycolic acid, which have no affinity for muscarinic receptors. Tiotropium, even at concentrations above therapeutic levels, does not inhibit cytochrome P450 1A1, 1A2, 2B6, 2C9, 2C19, 2D6, 2E1 and 3A. The final elimination half-life occurs on the 5th–6th day after inhalation. After inhalation in powder form, 14% of the dose is excreted in the urine, the rest is not absorbed in the intestine and is excreted in the feces. The renal clearance of tiotropium bromide exceeds the clearance of creatinine, indicating urinary excretion. After continuous daily inhalation in patients with COPD, a steady state is achieved within 2–3 weeks without subsequent cumulation. Tiotropium demonstrated linear pharmacokinetic properties in the therapeutic range after dry powder inhalation. Pharmacokinetics in the Elderly As with all drugs that are primarily excreted in the urine, tiotropium in the elderly is associated with decreased renal clearance due to decreased renal function (326 mL/min in patients with COPD over 58 years of age versus 163 ml/min in patients with COPD over the age of 70 years). The excretion of tiotropium in the urine after inhalation decreases from 14% (in healthy young volunteers) to 7% (in elderly patients with COPD), but the plasma concentration does not change significantly in elderly patients with COPD and does not exceed the interindividual and interindividual limits. individual variability (43% increase in AUC after dry powder inhalation). Pharmacokinetics in patients with impaired renal function In patients with renal failure, the concentration of the drug in the blood plasma increases, and renal clearance decreases. With mild renal failure (creatinine clearance 50–80 ml/min), the concentration of tiotropium bromide in the blood plasma increases slightly (increase in AUC value after IV infusion by 39%). Pharmacokinetics in patients with impaired liver function Liver failure does not have a significant effect on the pharmacokinetics of the drug. Tiotropium is mostly excreted by renal elimination (up to 74% in healthy young volunteers) and by simple non-enzymatic cleavage of the ester to products that do not bind to muscarinic receptors.
Side effects
Inhalation of Spiriva and Spiriva Respimat may be accompanied by:
- rash;
- dry mouth;
- candidiasis of the oral mucosa;
- bronchospasm;
- dysphonia;
- tachycardia;
- dizzy;
- itching;
- hives;
- dysphagia;
- constipation;
- gastroesophageal reflux ;
- cough;
- supraventricular tachycardia ;
- throat irritation;
- increased intracranial pressure ;
- intestinal obstruction;
- difficulty urinating;
- anaphylaxis;
- nosebleeds;
- urinary tract infections;
- atrial fibrillation;
- blurred vision;
- glaucoma;
- angioedema.
Spiriva, instructions for use
Instructions for use of Spiriva in capsule form suggest their use in the form of inhalation using the HandiHaler device (inhaler). The recommended daily dose is one capsule. The use of Spiriva capsules orally (internally) is not allowed.
The medicine Spiriva Respimat in the form of a solution is also intended for inhalation using the Respimat inhaler. The recommended daily dose is 5 mcg (2 inhalations) once at one time of the day.
Elderly patients and patients with liver pathologies take drugs without dose adjustment.
For kidney disease, dosage adjustment is also not required, but regular testing of kidney function is advisable.
For inhalation of the drug Spiriva in the form of capsules, a special inhaler HandiHaler has been developed, which should not be used for other drugs. The service life of this inhaler is 12 months.
The inhaler kit includes: mouthpiece, dust cap, base, central chamber and lancing button.
When using it you should:
- open the dust cap by pressing the piercing button;
- lifting up, open the cap completely, and then the mouthpiece in the same way;
- place the capsule in the central chamber (either side), after removing it from the package;
- Close the mouthpiece thoroughly (you will hear a characteristic click) without closing the dust cap;
- with the inhaler positioned with the mouthpiece up, press the piercing button one full time and release (to create a hole in the capsule);
- exhale as completely as possible (not into the mouthpiece of the inhaler);
- place the inhaler in the oral cavity, tightly covering the mouthpiece with your lips;
- With the head positioned straight, take a deep, slow, but quite strong breath (while hearing or feeling the vibration of the capsule) until the lungs are completely saturated;
- hold your breath until you feel discomfort, while removing the inhaler from your mouth;
- repeat similar breaths until the contents of the capsule are completely released;
- open the mouthpiece and remove the capsule, then close the mouthpiece and dust cap.
The HandiHaler inhaler should be cleaned once every 30 days.
To do this, after first lifting the piercing button, you need to open the dust cap and mouthpiece, as well as the base of the device. Using warm water, rinse the inhaler until any remaining powder is removed. Wipe with a paper towel and let air dry for 24 hours. If necessary, you can clean the outer surface of the mouthpiece using a damp cloth.
When removing a capsule from a blister, separate its strip along the perforation line. Open the capsule bed and remove it before use. Do not allow the removed capsule to remain in the open air for a long time.
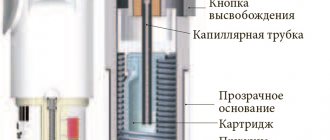
To inhale the drug Spiriva Respimat, use the Respimat inhaler:
- the first opening is carried out by displacing the transparent base by pressing the safety lock, with the green cap closed;
- remove the cartridge from the packaging and insert the narrow end into the inhaler, a click should be heard (do not remove the cartridge after installing it);
- install the transparent base in its original place;
- holding the inhaler in your right hand, turn its base until it clicks, following the direction of the red arrows (half a turn);
- open the green cap completely;
- pointing the inhaler down, press the button until the drug is released, then close the cap.
After this, the inhaler is charged and ready for further use.
To carry out inhalations you should:
- with the green cap closed, holding the inhaler in your right hand, turn its base until it clicks, following the direction of the red arrows (half a turn);
- open the green cap completely;
- exhale slowly and completely, then tightly cover the mouthpiece with your lips without closing the ventilation holes;
- pointing the mouthpiece towards the back wall of the throat, take a deep, slow breath, while pressing the inhaler button (continue inhalation as long as possible);
- hold your breath for at least 10 seconds;
- repeat the procedure until the full dose is received.
Inhalations are carried out once every 24 hours, after which the cap is closed.
If the inhaler is not used for a week, it is necessary to perform a preventive spray with the mouthpiece pointing down.
The Respimat inhaler is designed for 60 breaths and is equipped with a dose indicator. The indicator reading at the beginning of the red zone indicates a 7-day supply of the drug (14 breaths). This is a reason to purchase a new inhaler.
The inhaler can be used for 3 months, after which, even if not fully used, it must be thrown away.
Clean the mouthpiece once a week using a damp cloth.
Spiriva Respimat solution for inhalation 2.5 mcg/dose 4 ml cart.
Registration Certificate Holder
BOEHRINGER INGELHEIM INTERNATIONAL (Germany)
Dosage form
Medicine - Spiriva® Respimat®
Description
Solution for inhalation
transparent, colorless or almost colorless.
1 inhalation dose
tiotropium bromide monohydrate 3.1235 mcg, which corresponds to a tiotropium content of 2.5 mcg
Excipients
: benzalkonium chloride - 1.105 mcg, disodium edetate - 1.105 mcg, hydrochloric acid 1M - up to pH 2.8-3.0, water - up to 11.05 mg.
60 inhalation doses (30 therapeutic doses) - cartridges with a capacity of 4.5 ml (1), placed in an aluminum cylinder, complete with the Respimat® inhaler - cardboard packs.
Indications
- for maintenance treatment of patients with COPD, chronic bronchitis, emphysema; maintenance therapy for persistent shortness of breath; improving the quality of life impaired due to COPD and reducing the frequency of exacerbations;
- as an additional maintenance therapy in patients over 6 years of age with bronchial asthma, with persistent symptoms of the disease while taking at least inhaled corticosteroids, to reduce symptoms of bronchial asthma, improve quality of life and reduce the frequency of exacerbations.
Contraindications for use
- hypersensitivity to the components of the drug, to atropine or its derivatives: to ipratropium bromide, oxitropium bromide.
Carefully
the drug should be used for angle-closure glaucoma, prostatic hyperplasia, bladder neck obstruction.
pharmachologic effect
Tiotropium bromide is a long-acting m-anticholinergic blocker. The drug has the same affinity for the M1-M5 subtypes of muscarinic receptors. The result of inhibition of M3 cholinergic receptors in the airways is relaxation of smooth muscles. The bronchodilator effect depends on the dose and lasts for at least 24 hours. The significant duration of action is probably due to the very slow dissociation of the drug from M3 cholinergic receptors; The half-dissociation period is significantly longer than that of ipratropium bromide.
When administered by inhalation, tiotropium bromide, as an N-quaternary ammonium derivative, has a local selective effect (on the bronchi), while in therapeutic doses it does not cause systemic m-anticholinergic side effects. Dissociation from M2 cholinergic receptors occurs faster than from M3 cholinergic receptors, which indicates the predominance of selectivity for the M3 receptor subtype over M2 cholinergic receptors. High affinity for receptors and slow dissociation of the drug from connection with receptors determine a pronounced and long-lasting bronchodilator effect in patients with COPD.
Bronchodilation that develops after inhalation of tiotropium bromide is primarily due to local (on the respiratory tract) rather than systemic effects.
Clinical studies have shown that the use of Spiriva® Respimat® 1 time / day leads to a significant improvement (compared to placebo) in lung function (FEV1 and FVC) within 30 minutes after the first dose. Improvement in lung function persists for 24 hours at steady state.
Pharmacodynamic equilibrium was achieved within one week. Spiriva® Respimat® significantly improved morning and evening peak expiratory volume flow (PEEF) measured by patients. Spiriva® Respimat® resulted in a reduction (compared with placebo) in rescue bronchodilator use. The bronchodilator effect of the drug persists for 48 weeks of use of the drug; There are no signs of addiction.
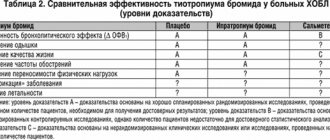
Analysis of combined data from two randomized, placebo-controlled, crossover clinical trials showed that the bronchodilator effect of Spiriva® Respimat® (5 mcg) after a 4-week treatment period was quantitatively greater than the effect of Spiriva® (18 mcg).
In long-term (12-month) studies, Spiriva® Respimat® was found to significantly reduce shortness of breath; improves quality of life; reduces the psychosocial impact of COPD and increases activity.
Spiriva® Respimat® significantly improved overall health status (total score) compared with placebo at the end of two 12-month studies, and this difference was maintained throughout the treatment period; Spiriva® Respimat® significantly reduced the number of exacerbations of COPD and increased the period until the first exacerbation compared to placebo.
Spiriva® Respimat® has been shown to reduce the risk of exacerbation of COPD and significantly reduce the number of hospitalizations.
In a retrospective analysis of individual clinical studies, a statistically insignificant increase, compared with placebo, in the number of deaths in patients with cardiac arrhythmias was observed. However, these data are not statistically confirmed and may be associated with heart disease.
In clinical studies in patients suffering from bronchial asthma and continuing to experience symptoms of the disease despite maintenance therapy with inhaled corticosteroids, incl. in combination with a long-acting beta2-adrenergic receptor agonist, it was found that the addition of Spiriva® Respimat® to maintenance therapy led to a significant improvement in lung function compared with placebo, significantly reduced the number of serious exacerbations and periods of worsening symptoms of bronchial asthma, and increased the period to the first their onset led to a significant improvement in the quality of life and an increase in the number of patients with a positive response to maintenance therapy.
The bronchodilator effect of the drug persisted throughout 1 year of use, and no signs of addiction were observed.
Drug interactions
Although specific drug interaction studies have not been conducted, tiotropium bromide has been used in conjunction with other drugs used to treat COPD, including sympathomimetic bronchodilators, methylxanthines, oral and inhaled corticosteroids, antihistamines, mucolytics, leukotriene modifiers, cromones, anti-IgE drugs; however, there were no clinical signs of drug interaction.
Combined use with long-acting beta2-agonists, inhaled corticosteroids and their combinations does not affect the effect of tiotropium
Long-term combined use of tiotropium bromide with other m-anticholinergic drugs has not been studied. Therefore, long-term combined use of Spiriva® Respimat® with other m-anticholinergic drugs is not recommended.
Dosage regimen
The recommended therapeutic dose is 2 inhalations of the Respimat® inhaler spray (5 mcg/therapeutic dose) 1 time/day, at the same time of day.
When treating bronchial asthma, the full therapeutic effect occurs within a few days.
In elderly patients, patients with impaired liver function and patients with minor impaired renal function (creatinine clearance 50-80 ml/min)
you can use the drug Spiriva® Respimat® at the recommended dose.
However, the use of the drug in patients with moderate or significant renal impairment (creatinine clearance <50 ml/min)
should be carried out under close supervision.
COPD does not usually occur in children. Safety and effectiveness of Spiriva® Respimat® in children under 1 year of age
have not been studied.
Rules for using the Spiriva® Respimat® inhaler
Before you start using the drug, you should study the rules for using the Spiriva® Respimat® inhaler.
The inhaler is intended for use once a day. Each time you use it, you should take 2 inhalations.
img_spiriva_respimat_1.tif|png
How to store Spiriva® Respimat® inhaler
Keep Spiriva® Respimat® out of the reach of children.
Do not freeze Spiriva® Respimat®.
If the Spiriva® Respimat® inhaler has not been used for more than 7 days, you should point it down before use and press the dose button once.
If the Spiriva® Respimat® inhaler has not been used for more than 21 days, you should repeat steps 4-6 from the “Preparation for first use” section until a cloud of aerosol appears. Then repeat steps 4-6 three more times.
Do not use Spiriva® Respimat® inhaler after the expiration date.
Do not touch the piercing element inside the transparent sleeve.
Caring for your Spiriva® Respimat® inhaler
The mouthpiece and the metal part inside the mouthpiece should be cleaned with a damp cloth or cloth at least once a week.
Any slight change in the color of the mouthpiece does not affect the operation of the Spiriva® Respimat® inhaler.
Determining when to start using a new inhaler
img_spiriva_respimat_2.tif|png
The Spiriva® Respimat® inhaler contains 60 inhalation doses (i.e. 30 therapeutic doses) when used in accordance with the dosing regimen (2 inhalation doses 1 time / day).
The dose indicator shows approximately how many doses are left. When the indicator points to the red area of the scale, it means there is approximately 7 days of medication left (14 inhalation doses).
When the dose indicator of the inhaler reaches the end of the red scale, the Spiriva® Respimat® inhaler will automatically lock - no more inhalation doses can be delivered (turning the transparent sleeve will not be possible).
The Spiriva® Respimat® inhaler should be thrown away 3 months after first use, even if it is not completely used.
Preparing for first use
1. Remove the transparent sleeve
img_spiriva_respimat_3.tif|png
- Keep cap closed.
- Press the locking button and pull firmly on the transparent sleeve with your other hand.
2. Insert cartridge
img_spiriva_respimat_4.tif|png
- Insert the cartridge with the narrow end into the inhaler.
- Place the inhaler with the bottom of the cartridge on a hard surface and press firmly until the cartridge clicks into place.
3. Replace the transparent sleeve
img_spiriva_respimat_5.tif|png
- Place the transparent sleeve in place until it clicks.
4. Rotate
img_spiriva_respimat_6.tif|png
- Keep cap closed.
- Turn the clear sleeve in the direction indicated by the arrows on the label until you hear a click (half a turn).
5. Open
img_spiriva_respimat_7.tif|png
- Open the cap all the way.
6. Click
img_spiriva_respimat_8.tif|png
- Point the inhaler downwards.
- Press the dose button.
- Close the cap.
- Repeat steps 4-6
until a cloud of aerosol appears. - After a cloud of aerosol appears,
repeat steps 4-6 3 more times.
Daily use
To turn
img_spiriva_respimat_9.tif|png
- Keep cap closed.
- Turn
the transparent sleeve in the direction indicated by the arrows on the label until it clicks (half a turn).
Open
img_spiriva_respimat_10.tif|png
- Open
the cap all the way.
Click
img_spiriva_respimat_11.tif|png
Exhale slowly and completely.
Place your lips around the mouthpiece without blocking the air intakes.
Taking a slow, deep breath through your mouth, press
dose button and continue to inhale.
Hold your breath for about 10 seconds or as long as possible.
To receive the second inhalation dose, repeat the following steps: Turn, Open, Press.
Answers to frequently asked questions
1. It is difficult to install the cartridge to the required depth
Did you accidentally turn the clear sleeve before installing the cartridge?
Open the cap, press the dose button, then insert the cartridge.
Are you inserting the cartridge with the wide end?
Insert the narrow end of the cartridge into the inhaler.
2. It is impossible to press the dose button
Have you turned the transparent sleeve?
If not, turn the clear sleeve in one continuous motion until it clicks (half a turn).
Does the dose indicator of the Spiriva® Respimat® inhaler indicate zero?
The Spiriva® Respimat® inhaler is blocked after the release of 60 inhalation doses (30 therapeutic doses). Prepare and use your new Spiriva® Respimat® inhaler.
3. The transparent sleeve cannot be rotated
Have you turned the clear sleeve yet?
If the clear sleeve is already rotated, follow the "Open" and "Push" steps in the "Daily Use" section to obtain the inhalation dose.
Does the dose indicator of the Spiriva® Respimat® inhaler indicate zero?
The Spiriva® Respimat® inhaler is blocked after the release of 60 inhalation doses (30 therapeutic doses). Prepare and use your new Spiriva® Respimat® inhaler.
4. The dose indicator of the Spiriva® Respimat® inhaler reaches zero too quickly
Have you used Spiriva® Respimat® in accordance with the dosage regimen (two inhalation doses once a day)?
The drug Spiriva® Respimat® lasts for 30 days when using two inhalations 1 time per day.
Did you rotate the clear sleeve before installing the cartridge?
The dose indicator counts every turn of the transparent sleeve, regardless of whether the cartridge is installed or not.
Have you released inhalation doses into the air to test the performance of Spiriva® Respimat®
. Once the inhaler is prepared for use, daily inhalation checks are not required.
Have you installed a cartridge into a used Spiriva® Respimat® inhaler?
Always install a new cartridge in a new one
Spiriva® Respimat® inhaler.
5. The Spiolto® Respimat® inhaler releases inhalation doses automatically
Was the cap open when you turned the clear sleeve?
Close the cap, then rotate the clear sleeve.
Did you press the dose button while turning the transparent sleeve?
Close the cap so that the dose button is closed, then rotate the transparent sleeve.
Have you stopped while turning the clear sleeve until you heard a click?
Turn the clear sleeve in one
continuous
motion until it clicks (half a turn).
6. Spiriva® Respimat® inhaler does not release an inhalation dose
Have you installed the cartridge?
If not, install the cartridge.
Did you repeat the "Rotate", "Open", "Press" steps less than three times after installing the cartridge?
Repeat the "Rotate", "Open", "Push" steps three times after installing the cartridge as described in the "Preparing for First Use" section, steps 4-6.
Does the dose indicator of the Spiriva® Respimat® inhaler indicate zero?
If the dose indicator points to zero, the inhaler is empty and blocked.
Do not remove the clear sleeve or cartridge after preparing the inhaler for use. Always install a new cartridge in a new one
Spiriva® Respimat® inhaler.
Overdose
When using the drug in high doses, manifestations of m-anticholinergic action are possible. After 14 days of inhaled use of tiotropium bromide in doses reaching 40 mcg, no significant adverse events were observed in healthy individuals, except for a feeling of dryness of the mucous membranes of the nose and oropharynx, the frequency of which depended on the dose (10-40 mcg/day). The exception was a clear decrease in salivation starting from the 7th day of drug use. In six long-term studies in patients with COPD, no significant adverse events were observed with inhaled tiotropium bromide solution at a daily dose of 10 mcg for 4-48 weeks.
Side effect
Many of the adverse reactions listed below may be due to the m-anticholinergic properties of the drug.
Adverse reactions were identified based on data obtained during clinical trials and individual reports during post-registration use of the drug.
Definitions of categories of frequency of adverse reactions: very often (≥1/10), often (≥1/100, <1/10), infrequently (from ≥1/1000, <1/100), rarely (≥1/10,000, <1/1000), very rare (<1/10,000), frequency unknown (frequency cannot be estimated from available data).
From the side of metabolism:
frequency unknown - dehydration.
From the nervous system:
infrequently - dizziness; rarely - insomnia.
From the side of the organ of vision:
rarely - increased intraocular pressure, glaucoma, blurred vision.
From the cardiovascular system:
rarely - atrial fibrillation, tachycardia (including supraventricular tachycardia), palpitations.
From the respiratory system:
infrequently - cough, pharyngitis, dysphonia; rarely - nosebleeds, bronchospasm, laryngitis; frequency unknown - sinusitis.
From the digestive system:
often - slight transient dryness of the pharyngeal mucosa; uncommon - constipation, oral candidiasis; rarely - dysphagia; rarely, gastroesophageal reflux, gingivitis, glossitis; frequency unknown - stomatitis; intestinal obstruction, including paralytic ileus.
For the skin and subcutaneous tissues:
rarely - skin infections and skin ulcers, dry skin.
Allergic reactions:
uncommon - rash, itching;
rarely - angioedema, urticaria; frequency unknown - hypersensitivity, including immediate reactions. From the musculoskeletal system:
frequency unknown - swelling of the joints.
From the kidneys and urinary system:
uncommon - dysuria, urinary retention (more often in men with predisposing factors); rarely - urinary tract infection.
special instructions
The drug Spiriva® Respimat®, as a bronchodilator used once a day for maintenance treatment, should not be used as initial therapy for acute attacks of bronchospasm or to eliminate acute symptoms. In case of an acute attack, fast-acting beta2-agonists are used.
Spiriva® Respimat® should not be used for the treatment of bronchial asthma as first-line therapy. Patients should be advised to continue anti-inflammatory therapy (for example, inhaled corticosteroids) while taking Spiriva® Respimat®, even if symptoms decrease.
Immediate hypersensitivity reactions may occur after using the drug.
Inhalation of the drug may cause bronchospasm.
In case of moderate or severe renal failure (creatinine clearance ≤50 ml/min), the drug should be taken under close supervision, as with all drugs excreted primarily by the kidneys.
Before use, patients should be familiar with the instructions for use.
Do not allow the solution or aerosol to come into contact with your eyes. Pain or discomfort in the eyes, blurred vision, visual halos combined with redness of the eyes, swelling of the conjunctiva and cornea can be symptoms of acute angle-closure glaucoma. If any combination of these symptoms develops, you should immediately consult a specialist. Miotic eye drops are not considered an effective treatment.
Spiriva® Respimat® should not be used more than once a day.
Spiriva® cartridges should only be used with the Respimat® inhaler.
Impact on the ability to drive vehicles and machinery
No studies have been conducted to study the effect on the ability to drive vehicles and machinery. Care should be taken when performing these activities as... Dizziness or blurred vision may develop.
Storage conditions
The drug should be stored out of the reach of children at a temperature not exceeding 25°C. Do not freeze.
Best before date
Shelf life: 3 years.
Use within 3 months after the first inhalation.
Use during pregnancy and breastfeeding
Restrictions during pregnancy - Contraindicated. Restrictions when breastfeeding - Contraindicated.
Data on the effect of Spiriva® Respimat® on pregnancy are limited. In preclinical studies studying reproductive toxicity, there was no indication of direct or indirect adverse effects of the drug. As a precaution, it is preferable to avoid using Spiriva® Respimat® during pregnancy.
There are no clinical data on the effects of tiotropium bromide during breastfeeding.
The drug should not be used in pregnant or breastfeeding women unless the potential benefit to the mother outweighs the potential risk to the fetus and child. During the period of use of the drug, it is necessary to stop breastfeeding.
Use for renal impairment
Restrictions for impaired renal function - With caution.
In patients with minor renal impairment (creatinine clearance 50-80 ml/min)
you can use the drug Spiriva® Respimat® in the recommended dose.
However, the use of the drug in patients with moderate or significant renal impairment (creatinine clearance <50 ml/min)
should be carefully monitored.
Use for liver dysfunction
Restrictions for liver dysfunction - No restrictions. In patients with liver dysfunction
you can use the drug Spiriva® Respimat® at the recommended dose.
Use in elderly patients
Restrictions for elderly patients - No restrictions. For elderly patients
you can use the drug Spiriva® Respimat® at the recommended dose.
Use in children
Restrictions for children - Contraindicated.
Contraindicated for use in children and adolescents under 18 years of age.
(due to lack of data on efficacy and safety).
Terms of sale
The drug is available with a prescription.
Contacts for inquiries
BERINGER INGELHEIM INTERNATIONAL GmbH (Germany)
Boehringer Ingelheim LLC
125171 Moscow Leningradskoe highway 16A, building 3 Tel. Fax
special instructions
The drugs Spiriva and Spiriva Respimat are not used to treat bronchospasm , especially during acute attacks.
Bronchospasm and immediate hypersensitivity may occur immediately after inhalation.
The patient must be provided with complete information on the use of a particular inhaler. Drugs should not be allowed to come into contact with the eyes. Eye discomfort, pain , blurred vision , redness , corneal swelling , conjunctival congestion may indicate an acute attack of angle-closure glaucoma . If you observe any of these symptoms, you should inform your doctor.
Special instructions for the use of Spiriva
During pregnancy and breastfeeding. There are no clinical data regarding the safety of the use of Spiriva during pregnancy, however, experimental studies have not revealed any direct or indirect effect on the course of pregnancy and the development of the embryo/fetus, childbirth and postnatal development. There are no clinical data regarding the safety of Spiriva during breastfeeding, but it has been experimentally established that a small amount of tiotropium bromide passes into breast milk. The drug should not be used during pregnancy and lactation without careful assessment of the expected benefit to the mother and the potential risk to the fetus or child. Based on the fact that there is no experience with the use of Spiriva in the treatment of children, the drug is recommended for use only in adults. Spiriva is a bronchodilator that is prescribed once a day for maintenance therapy and is not used to relieve acute bronchospasm attacks. As with other anticholinergic drugs, Spiriva should be used with caution in patients with angle-closure glaucoma, prostatic hyperplasia, or bladder neck obstruction. Inhaled drugs can cause the development of paradoxical (inhalation-induced) bronchospasm. Patients should be instructed regarding the correct use of Spiriva capsules. Do not allow the powder to get into your eyes, which can provoke an attack of angle-closure glaucoma. Signs of angle-closure glaucoma may include pain or discomfort in the eyes, blurred vision, the appearance of a halo around objects or colored spots in front of the eyes in combination with conjunctival or corneal hyperemia. Consultation with an ophthalmologist is required as the use of miotic eye drops may not be effective. Spiriva should not be used more than once a day. Spiriva capsules should only be used with the HandyHaler device. The drug contains 5.5 mg of lactose monohydrate per capsule. Impact on the ability to drive vehicles and operate mechanical devices. No research was carried out. Dizziness or blurred vision may affect your ability to operate vehicles or use mechanical equipment.
Analogues of Spiriva and Spiriva Respimat
Level 4 ATX code matches:
Atrovent
The most used analogues of these drugs are:
- Atrovent;
- Ipravent;
- Truvent;
- Troventol;
- Ipratropium;
- Sibri Breezhaler.
The price of analogues depends on the active substance of the drug, the manufacturer, the number of doses and many other factors. For example, inhalation Atrovent can be purchased for 350 rubles, while the cost of Sibri Breezhaler varies within 2000 rubles.
Reviews
Reviews about Spiriva, as well as reviews about Spiriva Respimat, are almost 100% positive. According to many doctors, these medications are the “golden mean” when conducting maintenance therapy for COPD . The high effectiveness and duration of action of the drugs is also confirmed by the patients using them.
bronchial asthma showed good results , but only as an additional means to alleviate the general condition.
Some problems arise when using inhalers. For proper use, you should exactly repeat the procedure described in the instructions for use of the inhalers, and also carry out several inhalations in the presence of an experienced doctor who can correct errors that occur.
Drug interactions Spiriva
Although formal drug interaction studies have not been conducted, tiotropium bromide has been used concomitantly with other drugs (sympathomimetic bronchodilators, methylxanthines, oral and inhaled steroids used in the treatment of COPD) without developing side effects. There is insufficient information on the use of other anticholinergic drugs in combination with Spiriva: a single dose of ipratropium bromide during long-term use of Spiriva in patients with COPD and healthy volunteers was not associated with an increase in the number of adverse reactions, changes in vital signs or ECG results. The use of Spiriva in combination with other medicinal products containing anticholinergics has not been studied and is therefore not recommended.
Spiriva price, where to buy
In Russia, the price of Spiriva 18 mcg capsule No. 30 is approximately 2500 - 2800 rubles.
You can buy Spiriva Respimat for 3000 – 3600 rubles.
The price in Ukraine is about 800 hryvnia for 30 capsules and about 1000 hryvnia for a solution.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Spiriva capsules for inhalation.
18 mcg 30 pcs. Boehringer Ingelheim RUB 2,068 order - Spiriva Respimat solution for inhalation. 2.5 µg/dose 4 ml Boehringer Ingelheim
RUR 2,086 order
Pharmacy Dialogue
- Spiriva respimat (d/ing solution 2.5 mcg/dose 4 ml cartridges 60 doses) Boehringer Ingelheim
RUB 1,968 order
- Spiriva (caps. d/ing. 18 µg No. 30 with HandiHaler inhaler) Boehringer Ingelheim
RUR 1,971 order
show more
Pharmacy24
- Spiriva 18 mcg No. 30 capsules + Handy Highler device 60% discount Boehringer Ingelheim Pharma GmbH i Co.
KG, Nimechchina 618 UAH. order - Spiriva Respimat 2.5 mcg 4 ml No. 1 solution + Respimat inhaler
1661 UAH. order
- Spiriva 18 mcg No. 30 + Handy Highler device capsules Boehringer Ingelheim Pharma GmbH & Co. KG, Nimechchina
1476 UAH order
- Spiriva Respimat 2.5 mcg 4 ml solution + Respimat inhaler 67% discount
743 UAH order
PaniPharmacy
- Spiriva Respimat liquid Spiriva Respimat solution for inhalation 2.5 µg 4 ml 60 doses Germany, Boehringer Ingelheim Pharma
1285 UAH. order
- Spiriva capsules 18mkg No. 30 Australia, Boehringer Ingelheim
1727 UAH. order
- Spiriva capsule Spiriva capsules 18 µg with Handy Hayler device No. 30 Germany, Boehringer Ingelheim Pharma
1594 UAH. order
show more