Valparine® XR
Before starting to use the drug and periodically during the first 6 months of treatment, especially in patients at risk of developing liver damage, liver function tests should be performed.
As with the use of most antiepileptic drugs, when using valproic acid, a slight increase in the activity of “liver” transaminases is possible, especially at the beginning of treatment, which occurs without clinical manifestations and is transient. In such patients, it is necessary to conduct a more thorough study of biological parameters, including the prothrombin index. It may be necessary to adjust the dose of the drug, and, if necessary, repeat clinical and laboratory examination.
Before starting therapy or before surgery, as well as in the event of spontaneous occurrence of subcutaneous hematomas or bleeding, it is recommended to determine the bleeding time and the number of formed elements in the peripheral blood, including platelets.
Severe liver damage
Predisposing factors
Isolated cases of severe liver damage, sometimes fatal, have been described. Clinical experience shows that patients taking multiple antiepileptic drugs at the same time and patients taking salicylates at the same time (since salicylates are metabolized through the same metabolic pathway as valproic acid) are at risk.
Suspicion of liver damage
For early diagnosis of liver damage, clinical observation of patients is mandatory. In particular, you should pay attention to the following symptoms that may precede the onset of jaundice, especially in patients at risk:
- nonspecific symptoms, especially those that began suddenly, such as asthenia, anorexia, lethargy, drowsiness, which are sometimes accompanied by repeated vomiting and abdominal pain;
- resumption of seizures in patients with epilepsy.
Patients or their family members (when using the drug in pediatric patients) should be warned that they should immediately report the occurrence of any of these symptoms to their doctor. Patients should immediately undergo clinical examination and laboratory testing of liver function tests.
Revealing
Liver function tests should be performed before starting treatment and then periodically during the first 6 months of treatment. Among conventional studies, the most informative are studies reflecting the state of the protein-synthetic function of the liver, especially the determination of the prothrombin index. Confirmation of deviation from the norm of the prothrombin index in the direction of its decrease, especially in combination with deviations from the norm of other laboratory parameters (a significant decrease in the content of fibrinogen and blood clotting factors, an increase in the concentration of bilirubin and an increase in the activity of “liver” transaminases), as well as the appearance of other symptoms indicating for liver damage, requires discontinuation of the drug. As a precaution, if patients were taking salicylates concomitantly, their use should also be discontinued.
Pancreatitis
There are rare reported cases of severe forms of pancreatitis in children and adults, which developed regardless of age and duration of treatment. Several cases of hemorrhagic pancreatitis have been observed with rapid progression of the disease from the first symptoms to death.
Children are at increased risk of developing pancreatitis, and this risk decreases with increasing age of the child. Risk factors for developing pancreatitis may include severe seizures, neurological disorders, or anticonvulsant therapy. Liver failure combined with pancreatitis increases the risk of death.
If severe abdominal pain, nausea, vomiting and/or anorexia occur, patients should be evaluated immediately. If pancreatitis is confirmed, in particular, with increased activity of pancreatic enzymes in the blood, the use of valproic acid should be discontinued and appropriate treatment should be started.
Suicidal thoughts and attempts
Suicidal ideation and suicide attempts have been reported in patients taking antiepileptic drugs for some indications. A meta-analysis of randomized placebo-controlled trials of antiepileptic drugs also showed a 0.19% increase in the risk of suicidal ideation and suicide attempts in all patients taking antiepileptic drugs (including a 0.24% increase in this risk in patients taking antiepileptic drugs for epilepsy), compared with their frequency in patients taking placebo. The mechanism of this effect is unknown.
Therefore, patients taking the drug should be constantly monitored for suicidal thoughts and suicide attempts, and if they occur, appropriate treatment should be provided. Patients and their caregivers are advised to immediately consult a physician if they experience suicidal thoughts or suicide attempts.
Carbapenems
The simultaneous use of carbapenems is not recommended (see section “Interaction with other drugs”, “With caution”).
Patients with established or suspected mitochondrial diseases Valproic acid may initiate or aggravate the manifestations of the patient's existing mitochondrial diseases caused by mutations in mitochondrial DNA, as well as in the nuclear gene encoding the mitochondrial enzyme γ-polymerase (POLG). In particular, in patients with congenital neurometabolic syndromes caused by mutations in the gene encoding γ-polymerase (POLG), such as patients with Alpers-Huttenlocher syndrome, valproic acid is associated with a higher incidence of acute liver failure and liver-related deaths. outcomes. The presence of diseases caused by defects in γ-polymerase can be assumed in patients with a family history or symptoms of such diseases, including encephalopathy of unknown origin, refractory epilepsy (focal, myoclonic), status epilepticus, mental and physical retardation, psychomotor regression, axonal sensorimotor neuropathy, myopathy, cerebellar ataxia, ophthalmoplegia or complicated migraine with visual (occipital) aura. In accordance with current clinical practice, testing for mutations in the polymerase γ gene (POLG) should be performed to diagnose such diseases (see section "Contraindications").
Paradoxical increase in the frequency and severity of seizures (including the development of status epilepticus) or the appearance of new types of seizures As with the use of other antiepileptic drugs, when taking valproic acid, instead of improvement, some patients experienced a reversible increase in the frequency and severity of convulsive seizures (including the development of status epilepticus) or the emergence of new types of seizures. If seizures worsen, patients should immediately consult their doctor (see section "Side Effects").
Female children and adolescents, women of childbearing potential and pregnant women
Pregnancy Prevention Program
Valproic acid has a high teratogenic effect; the use of valproic acid leads to a high risk of congenital malformations and developmental disorders of the central nervous system in the fetus.
The use of valproic acid is contraindicated:
- during pregnancy for epilepsy, except in cases of absence of alternative treatment methods (see sections “Special instructions”, “Use during pregnancy and breastfeeding”);
— during pregnancy in the treatment and prevention of bipolar affective disorders;
- in women of childbearing potential, unless all the conditions of the Pregnancy Prevention Program are met (see sections “Special Instructions”, “Use during Pregnancy and Breastfeeding”).
When prescribing drugs containing valproic acid, you must:
— conduct an individual assessment of the circumstances of prescribing the drug in each individual case, discuss possible methods of therapy and make sure that the patient understands the potential risks and the need for measures taken to minimize them;
— make sure that the patient has childbearing potential;
— make sure that the patient understands the nature and magnitude of the risks of using valproic acid during pregnancy, in particular, the risks of teratogenic effects, as well as the risks of disorders of the mental and physical development of the child;
— make sure that the patient understands the need to conduct a pregnancy test before starting and during treatment;
- explain the necessary methods of contraception, make sure that the patient uses reliable methods of contraception continuously during treatment with drugs containing valproic acid;
— make sure that the patient understands the need to regularly contact a specialist in the treatment of epilepsy and bipolar affective disorders (at least once a year) to re-analyze the prescribed therapy;
- make sure that the patient understands the need to contact her doctor if she is planning a pregnancy in order to promptly assess the possibility of switching to alternative therapy before stopping the use of contraception;
- inform about the need for immediate consultation with your doctor if you suspect pregnancy;
— ensure that the patient has received all the necessary explanations about the risks and necessary precautions.
The above information is also relevant for women who are not currently sexually active, unless the attending physician is satisfied that there is no childbearing potential.
Female pediatric patients
When prescribing drugs containing valproic acid, you must:
— make sure that female pediatric patients/their legal representatives understand the need to consult with their doctor upon the onset of menarche;
— ensure that female pediatric patients who have reached menarche, or their legal representatives, receive detailed information about the risks of congenital malformations and disorders of the central nervous system in the fetus.
The treating physician should annually re-evaluate the prescribed valproic acid therapy and evaluate the possibility of prescribing alternative therapy. If drugs containing valproic acid are the treatment of choice, it is necessary to ensure that reliable methods of contraception are used and that the terms of the Pregnancy Prevention Program are followed. Before puberty, the possibility of switching patients to alternative treatment methods should be constantly considered.
Pregnancy test
Before starting treatment with drugs containing valproic acid, it is necessary to exclude pregnancy. Therapy with drugs containing valproic acid cannot be prescribed to women of childbearing potential unless a negative pregnancy test (pregnancy blood test) has been confirmed by a health care professional to prevent the drug from being prescribed during pregnancy.
Contraception methods
Female patients of childbearing potential who are prescribed therapy with drugs containing valproic acid should use reliable methods of contraception continuously throughout the entire treatment period.
Female patients of childbearing potential should be provided with detailed information about methods of preventing pregnancy, and such patients may also seek advice from their healthcare provider if they are not using a reliable method of contraception.
You must use at least one reliable method of contraception (preferably simultaneously with methods such as an intrauterine system or implant) or two complementary methods of contraception, including barrier methods. When prescribing a contraceptive method to a patient, it is necessary to take an individualized approach and discuss all possible contraceptive options with the patient to ensure that the patient adheres to and adheres to the regimen. In case of amenorrhea, the patient should also be warned about the use of effective methods of contraception.
Annual analysis of prescribed therapy
At least once a year, the treating physician should evaluate whether medications containing valproic acid are the treatment of choice. The risks associated with therapy should be discussed when prescribing the drug and at each annual review of the prescribed therapy, and ensure that the patient understands all risks.
Planning a pregnancy
If a patient is planning a pregnancy, a specialist in the treatment of epilepsy and bipolar affective disorder should evaluate therapy with drugs containing valproic acid and consider alternative therapy. Every effort should be made to switch the patient from therapy with drugs containing valproic acid before conception and until contraception is discontinued (see section “Use during pregnancy and breastfeeding”). If alternative therapy is not available, the patient should be advised of the risks associated with the use of drugs containing valproic acid for the unborn child to help make an informed decision about family planning.
What to do if you become pregnant?
If you become pregnant, you should contact your healthcare provider immediately to evaluate your treatment and consider alternative therapy.
The health worker must ensure that:
— patients understand all the risks described above;
— patients received recommendations not to stop therapy with valproic acid and to immediately contact their doctor when planning pregnancy.
Concomitant use with estrogen-containing drugs
Valproic acid does not reduce the therapeutic effectiveness of hormonal contraceptives. However, drugs containing estrogen, including estrogen-containing hormonal contraceptives, may increase the clearance of valproic acid, which may lead to a decrease in its serum concentration and, consequently, a decrease in its effectiveness. It is necessary to monitor the concentration of valproic acid in the blood serum and clinical effectiveness (seizure control and mood control) when prescribing or discontinuing estrogen-containing drugs (see section "Interaction with other drugs").
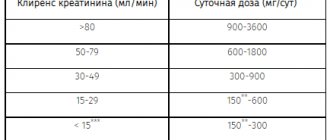
Kidney failure
It may be necessary to reduce the dose of valproic acid due to an increase in the concentration of its free fraction in the blood serum. If it is impossible to monitor plasma concentrations of valproic acid, the dose of the drug should be adjusted based on clinical observation of the patient.
Enzyme deficiency of the carbamide cycle (urea cycle)
If an enzymatic deficiency of the carbamide cycle is suspected, the use of valproic acid is contraindicated. In such patients, several cases of hyperammonemia with the development of stupor or coma have been described. In these cases, metabolic studies should be carried out before starting treatment with valproic acid (see section “Contraindications”). In children with unexplained gastrointestinal symptoms (anorexia, vomiting, cases of cytolysis), a history of lethargy or coma, with mental retardation or a family history of death of a newborn or child, metabolic studies should be carried out before starting treatment with valproic acid drugs, in particular determination of ammonemia (presence of ammonia and its compounds in the blood) on an empty stomach and after meals (see section “Contraindications”).
Patients with systemic lupus erythematosus
Although immune dysfunction is extremely rare during treatment with valproic acid, the potential benefits of their use must be weighed against the potential risks when administered to patients with systemic lupus erythematosus.
Weight gain
Patients should be warned about the risk of weight gain at the beginning of treatment and the need to take dietary measures to minimize this phenomenon.
Patients with diabetes mellitus
Given the possibility of adverse effects of valproic acid on the pancreas, when using the drug in patients with diabetes mellitus, blood glucose concentrations should be carefully monitored. When testing urine for the presence of ketone bodies in patients with diabetes, it is possible to obtain false-positive results, since valproic acid is excreted partially by the kidneys in the form of ketone bodies.
Patients infected with human immunodeficiency virus (HIV)
In vitro studies have shown that valproic acid stimulates HIV replication under certain experimental conditions. The clinical significance of this fact is unknown. In addition, the significance of data obtained from in vitro studies for patients receiving maximally suppressive antiretroviral therapy has not been established. However, these data should be taken into account when interpreting the results of continuous viral load monitoring in HIV-infected patients taking valproic acid.
Patients with existing carnitine palmitoyltransferase (CPT) type II deficiency
Patients with existing CPT type II deficiency should be warned of the increased risk of rhabdomyolysis when taking valproic acid.
Ethanol
During treatment with valproic acid, ethanol consumption is not recommended.
Other special instructions
The inert matrix of the drug (extended release drug), due to the nature of its excipients, is not absorbed in the gastrointestinal tract; after the release of the active substances, the inert matrix is excreted by the intestines.
Valparin hr tab p/o film prolong. 300 mg 100 pcs
Contraindicated combinations Mefloquine: risk of epileptic seizures in patients with epilepsy due to increased metabolism of valproic acid and convulsant mefloquine.
St. John's wort: danger of reducing the concentration of valproic acid in the blood plasma.
Combinations not recommended
Lamotrigine: increased risk of severe skin reactions including toxic epidermal necrolysis. In addition, the plasma concentration of lamotrigine increases (its metabolism in the liver is slowed down by valproic acid). If the combination is necessary, careful clinical and laboratory monitoring is required.
Combinations requiring special precautions
Carbamazepine: increased concentration of the active metabolite of carbamazepine in plasma with signs of overdose. In addition, a decrease in the concentration of valproic acid in plasma is associated with increased metabolism of valproic acid in the liver under the influence of carbamazepine. Recommended: clinical observation, determination of drug concentrations in plasma and, possibly, dose adjustment, especially at the beginning of treatment.
Carbapenems, monobactams: meropenem, panipenem, and, by extrapolation, aztreonam and imipenem: increased risk of seizures due to a decrease in the concentration of valproic acid in the blood plasma. Recommended: clinical observation, determination of drug concentrations in blood plasma; dose adjustment of valproic acid may be required during treatment with an antibacterial agent and after its discontinuation.
Felbamate: increased plasma concentrations of valproic acid with the risk of overdose. Recommended: clinical and laboratory monitoring and possible dose revision of valproic acid during treatment with felbamate and after its discontinuation).
Phenobarbital, primidone: increased plasma concentrations of phenobarbital and primidone with signs of overdose, usually in children. In addition, a decrease in the concentration of valproic acid in plasma associated with increased hepatic metabolism under the influence of phenobarbital or primidone. Recommended: clinical monitoring during the first 15 days of combination treatment with immediate reduction of the dose of phenobarbital or primidone at the first signs of sedation, determination of blood concentrations of both anticonvulsants.
Phenytoin: changes in the concentration of phenytoin in plasma, the risk of a decrease in the concentration of valproic acid associated with increased metabolism of valproic acid in the liver under the influence of phenytoin. Recommended: clinical monitoring with determination of plasma concentrations of both antiepileptic drugs and, if necessary, adjustment of their doses.
Topiramate: Risk of hyperammonemia or encephalopathy. Recommended: clinical and laboratory monitoring during the first month of treatment and in case of symptoms of ammonemia.
Neuroleptics, monoamine oxidase inhibitors (MAO inhibitors), antidepressants, benzodiazepines: Valproic acid potentiates the effect of psychotropic drugs such as neuroleptics, MAO inhibitors, antidepressants, benzodiazepines. Recommended: clinical monitoring and, if necessary, dose adjustment of the drug.
Cimetidine and erythromycin: the concentration of valproic acid in the blood plasma increases.
Zidovudine: Valproic acid may increase plasma concentrations of zidovudine, resulting in increased zidovudine toxicity.
Combinations to Consider
Nimodipine (oral and, by extrapolation, parenteral): increased hypotensive effect of nimodipine due to a decrease in its metabolism under the influence of valproic acid and increased concentration in the blood plasma.
Acetylsalicylic acid: increased effects of valproic acid due to an increase in its concentration in the blood plasma.
When used concomitantly with anticoagulants, vitamin K antagonists require careful monitoring of the prothrombin index.
Other forms of interaction
Valproic acid does not have an enzyme-inducing effect and therefore does not affect the effectiveness of hormonal contraceptives containing combinations of estrogen and progesterone.