Ketonal capsules 50 mg No. 25
Name
Ketonal caps 50 mg in bottle. No. 25 pack No. 1
Description
Blue and white capsules.
Main active ingredient
Ketoprofen
Release form
Capsules
Dosage
50mg
pharmachologic effect
Pharmacodynamics
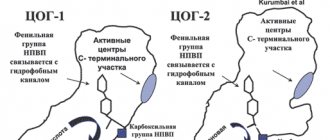
Ketonal is a non-steroidal antirheumatic drug with anti-inflammatory, analgesic and antipyretic effects. It is used for pain relief in a range of pain syndromes and for the treatment of inflammatory, degenerative and metabolic rheumatic diseases. Ketoprofen, the active ingredient of the drug, inhibits the synthesis of prostaglandins and leukotrienes by blocking the enzyme cyclooxygenase (cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2)), which catalyzes the synthesis of prostaglandins in the metabolism of arachidonic acid. Ketoprofen stabilizes lysosomal membranes in vitro and in vivo, in high concentrations inhibits the synthesis of leukotrienes in vitro and has anti-bradykinin activity in vivo. The mechanism of the antipyretic effect of ketoprofen is unknown. It is possible that ketoprofen inhibits the synthesis of prostaglandins in the central nervous system (most likely in the hypothalamus). In some women, ketoprofen reduces the symptoms of primary dysmenorrhea, probably by suppressing the synthesis and/or effectiveness of prostaglandins.
Pharmacokinetics
Ketoprofen is well absorbed from the gastrointestinal tract. After taking 100 mg of ketoprofen orally, its peak plasma concentration (10.4 mcg/ml) is reached in 1 hour 22 minutes. The bioavailability of ketoprofen after oral administration at a dose of 50 mg is 90% and increases linearly with increasing doses. Ketoprofen is a racemic mixture, but the pharmacokinetics of the two enantiomers are similar. 99% of ketoprofen binds to plasma proteins, mainly to the albumin fraction. The volume of distribution in tissues is 0.1-0.2 l/kg. Ketoprofen penetrates into the synovial fluid. Three hours after the administration of 100 mg of ketoprofen, its concentration in plasma is about 3 μg/ml, and the concentration in synovial fluid is 1.5 μg/ml. After nine hours, its concentration in plasma is about 0.3 μg/ml, and the concentration in synovial fluid is 0.8 μg/ml. This means that ketoprofen slowly penetrates into the synovial fluid and is also slowly eliminated from it, while its plasma concentration decreases further. If ketoprofen is taken with food, absorption slows down and plasma concentrations decrease slightly, but bioavailability remains the same. After oral administration of 50 mg ketoprofen with meals four times a day, a peak concentration of 3.9 μg/ml was achieved in 1.5 hours compared to 2.0 μg/ml after two hours when taking ketoprofen on an empty stomach. Equilibrium concentrations of ketoprofen are established 24 hours after its administration. In elderly patients, steady-state concentration was reached after 8.7 hours and was 6.3 mcg/ml. Ketoprofen is extensively metabolized by liver microsomal enzymes. It binds to glucuronic acid and is excreted in this form from the body. After oral administration, its plasma clearance is 1.16 ml/min/kg. Due to its rapid metabolism, its biological half-life is only two hours. Up to 80% of ketoprofen is excreted in the urine, mainly (over 90%) in the form of ketoprofen glucuronide, and about 10% is excreted in the feces. Special groups of patients With liver damage In patients with liver failure, probably due to hypoalbuminemia (free biologically active ketoprofen), the concentration of ketoprofen almost doubles, which requires the administration of a minimum daily dose that provides a sufficient therapeutic effect. With kidney damage In patients with renal failure, the clearance of ketoprofen is reduced. Therefore, in case of severe renal failure, a dose reduction is required.
Indications for use
Rheumatic diseases: - rheumatoid arthritis; - ankylosing spondylitis, cervical spondylitis; - osteoarthritis; - acute articular and chronic rheumatism (tendinitis, bursitis, capsulitis, synovitis). Pain: - pain in the lumbar region (muscle sprains/tears, lumbago, sciatica, fibrosis); - dysmenorrhea; - pain due to diseases of the musculoskeletal system.
Directions for use and doses
For oral administration. Capsules should be taken during or after meals with at least 100 ml of water or milk. Undesirable effects can be minimized by taking the drug at the lowest effective dose for the shortest possible time necessary to relieve symptoms. Recommended dose Adults • The usual dose is 1 capsule in the morning, afternoon and evening. • Dose for the treatment of rheumatoid arthritis and osteoarthritis: one capsule every 6 hours. • Dose for mild to moderate pain and dysmenorrhea: one capsule every 6-8 hours. Capsules can be combined with Ketonal suppositories. For example, one Ketonal capsule (50 mg) in the morning and afternoon and one Ketonal suppository (100 mg) in the evening. The maximum daily dose of ketoprofen is 200 mg. Before starting treatment at a dose of 200 mg per day, the possible risks and benefits should be carefully weighed. The use of higher doses is not recommended (see also “Precautions”). You can simultaneously take antacids, which reduce the likelihood of adverse effects of Ketonal on the digestive system. Elderly patients and patients with impaired renal function It is recommended to reduce the initial dose and prescribe maintenance therapy using the lowest effective dose. If the drug is well tolerated, dose adjustment can be considered on an individual basis. Patients with impaired liver function Such patients should be closely monitored and the drug should be used at the lowest effective daily dose. Children The drug is contraindicated for children (see “Contraindications”).
Use during pregnancy and lactation
Inhibition of prostaglandin synthesis may have a negative effect on pregnancy and/or embryo/fetal development. In the first and second trimesters of pregnancy, the drug should not be prescribed unless absolutely necessary. If Ketonal is used by a woman trying to become pregnant or in the first or second trimester of pregnancy, the dose should be as low as possible and the duration of treatment as short as possible. In the third trimester of pregnancy, the use of Ketonal is contraindicated. There are no data on the penetration of the drug into milk. It is not recommended to prescribe Ketonal to nursing mothers.
Precautionary measures
Avoid simultaneous use of the drug with NSAIDs, including selective COX-2 inhibitors. Older adults are more likely to experience adverse reactions to NSAIDs, especially gastrointestinal bleeding and perforation, which can be fatal (see Dosage and Administration). Gastrointestinal bleeding, ulceration, and perforation Gastrointestinal bleeding, ulceration, or perforation, which may be fatal, has been reported for all NSAIDs and may occur at any time during treatment, with or without preexisting symptoms or severe gastrointestinal disease. tract in the anamnesis. Taking Ketonal may be associated with a high risk of severe gastrointestinal toxicity, which is typical for some other NSAIDs, especially when taken in high doses (see also "Dosage and Administration" and "Contraindications"). The risk of gastrointestinal bleeding, ulceration or perforation increases with increasing doses of NSAIDs, in patients with a history of peptic ulcer disease, especially complicated by bleeding or perforation (see "Contraindications"), as well as in the elderly. Treatment of these patients should be started with the lowest dose available. For these patients, as well as for patients concomitantly taking low-dose acetylsalicylic acid or other drugs that increase the risk of gastrointestinal complications, combination therapy with protective drugs (eg, misoprostol or proton pump blockers) should be considered (see below and "Interaction with other drugs"). Patients with a history of gastrointestinal toxicity, especially the elderly, should report any unusual abdominal symptoms (especially gastrointestinal bleeding), especially early in treatment. Particular caution should be exercised when co-administered with drugs that may increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants (eg, warfarin), selective serotonin reuptake inhibitors, or antiplatelet agents such as acetylsalicylic acid (see Interactions with other medicines"). If patients experience gastrointestinal bleeding or ulcers during treatment with Ketonal, the drug should be discontinued. NSAIDs should be prescribed with caution to patients with a history of gastrointestinal diseases (ulcerative colitis, Crohn's disease), since they may experience exacerbations of these diseases (see “Side Effects”). Cardiovascular and cerebrovascular effects. Patients with a history of hypertension and/or mild to moderate congestive heart failure require appropriate monitoring and counseling as fluid retention and edema have been reported with the use of NSAIDs. The use of some NSAIDs (especially at high doses and during long-term treatment) may be associated with an increased risk of arterial thrombosis (eg, myocardial infarction or stroke) (see Precautions). There is insufficient data to exclude such a risk for ketoprofen. In patients with uncontrolled hypertension, congestive heart failure, established coronary artery disease, peripheral arterial disease and/or cerebrovascular disease, treatment with Ketonal should be carried out only after a careful assessment of benefits and risks. The same should be done before prescribing long-term treatment for patients with risk factors for cardiovascular disease (eg, hypertension, hyperlipidemia, diabetes mellitus, smoking). Patients suffering from bronchial asthma in combination with chronic rhinitis, chronic sinusitis and/or nasal polyposis are more likely to experience allergic reactions after taking acetylsalicylic acid and/or NSAIDs than other patients. Prescribing Ketonal may cause an attack of bronchial asthma or bronchospasm, especially in patients with an allergy to acetylsalicylic acid or NSAIDs (see “Contraindications”). In patients with heart failure, cirrhosis and nephrotic syndrome, as well as in patients taking diuretics and in patients with chronic renal failure, especially the elderly, renal function should be carefully monitored at the start of treatment. In such patients, the administration of Ketonal may cause a decrease in renal blood flow due to inhibition of prostaglandin synthesis and lead to decompensation of renal function. In patients with abnormal liver function tests or a history of liver disease, blood transaminase levels should be periodically monitored, especially during prolonged therapy. Rare cases of jaundice and hepatitis have been described in connection with the use of ketoprofen. Treatment should be discontinued if visual disturbances such as blurred vision occur. The drug is prescribed with caution to persons suffering from alcohol dependence. Severe skin reactions (some of them fatal) associated with the use of NSAIDs, such as exfoliative dermatitis, Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported extremely rarely (see “Side effects”). The greatest risk of developing these reactions is at the beginning of the course of treatment; in most cases, reactions occur in the first month of treatment. Ketonal should be discontinued at the first appearance of skin rash, lesions on the mucous membranes or other signs of hypersensitivity. Ketonal may mask signs and symptoms of infectious diseases, such as fever. Before extensive surgical interventions, the drug must be discontinued. The use of Ketonal may reduce fertility, so it is not recommended for women planning pregnancy. In women experiencing difficulty becoming pregnant or being evaluated for infertility, discontinuation of Ketonal should be considered. The drug contains lactose. Therefore, it should not be taken by patients with hereditary galactose intolerance, lactase deficiency or impaired absorption of glucose and galactose.
Interaction with other drugs
Drug combinations not recommended Other NSAIDs (including selective cyclooxygenase-2 inhibitors) and salicylates in high doses: increased risk of ulcers and bleeding in the gastrointestinal tract. Anticoagulants (heparin and warfarin) and platelet aggregation inhibitors (eg, ticlopidine, clopidogrel): increased risk of bleeding (see Precautions). If co-administration is necessary, close medical supervision is required. Lithium: Risk of increased plasma lithium levels, which can sometimes reach toxic levels due to decreased renal excretion of lithium. If necessary, plasma lithium concentrations should be carefully monitored and the dose of lithium adjusted during and after NSAID treatment. Methotrexate in doses exceeding 15 mg/week: increased risk of hematotoxicity of methotrexate, especially if used in high doses (>15 mg/week), which is likely due to the displacement of methotrexate from protein binding and reduced renal clearance. Combinations Requiring Caution Diuretics: Patients receiving diuretics, especially those with dehydration, are at increased risk of renal failure due to decreased renal blood flow due to inhibition of prostaglandin synthesis. Such patients should be adequately hydrated before initiating concomitant use of these drugs, and renal function should be monitored at the start of treatment (see Precautions). Angiotensin-converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor antagonists. In patients with impaired renal function (eg, dehydrated patients or the elderly), concomitant use of an ACE inhibitor or angiotensin II receptor antagonist and cyclooxygenase inhibitors may cause further deterioration of renal function, including possible acute renal failure. Methotrexate in doses below 15 mg/week: in the first weeks of combination treatment, it is necessary to monitor the detailed blood picture once a week. If there is any impairment of renal function and in elderly patients, monitoring should be carried out more often. Corticosteroids: Increased risk of developing ulcers or bleeding in the gastrointestinal tract (see Precautions). Pentoxifylline: increases the risk of bleeding. More frequent clinical monitoring and more frequent control of bleeding time are necessary. Combinations that need to be considered Antihypertensive drugs (beta blockers, ACE inhibitors, diuretics): ketoprofen reduces the effect of antihypertensive drugs (inhibition of the synthesis of vasodilator prostaglandins). Thrombolytics: increased risk of bleeding. Selective serotonin reuptake inhibitors: increased risk of gastrointestinal bleeding (see Precautions). Probenecid: Concomitant use of probenecid may significantly reduce the plasma clearance of ketoprofen. Combinations that should also be taken into account: Cyclosporine, tacrolimus: risk of additive nephrotoxicity, especially in elderly patients.
Contraindications
- hypersensitivity to ketoprofen or any of the excipients of the drug; - a history of rhinitis, bronchospasm, bronchial asthma, urticaria or allergic reactions after using ketoprofen or similar active substances, such as other nonsteroidal anti-inflammatory drugs (NSAIDs) or salicylates (eg, acetylsalicylic acid); in such patients, severe (in rare cases fatal) anaphylactic reactions have been described (see “Side effects”); - severe heart failure; — treatment of pain in the perioperative period during coronary artery bypass grafting (CABG); - history of chronic dyspepsia; - peptic ulcer in the acute phase, as well as a history of gastrointestinal bleeding, ulcer or perforation; - predisposition to bleeding; - severe renal dysfunction; - severe liver dysfunction; - last trimester of pregnancy (see “Pregnancy and lactation”); - children.
Compound
Each capsule contains 50 mg of ketoprofen. Excipients Capsule contents: lactose monohydrate, magnesium stearate, colloidal anhydrous silicon dioxide; capsule shell: gelatin, titanium dioxide E 171, blue dye VE 131.
Overdose
Cases of overdose of ketoprofen in a dose of up to 2.5 g have been described. In most cases, the observed symptoms were benign in nature and were limited to lethargy, drowsiness, nausea, vomiting and epigastric pain. There is no special antidote for an overdose of ketoprofen. If a significant overdose is suspected, gastric lavage and symptomatic and supportive therapy are recommended to eliminate dehydration. You also need to monitor diuresis and correct acidosis (if it develops). If renal failure develops, hemodialysis may be effective to remove the drug circulating in the blood.
Side effect
Edema, high blood pressure and heart failure have been reported in association with treatment with non-selective NSAIDs. If severe side effects occur, treatment should be discontinued. Adverse effects are distributed by organ system class, by frequency of occurrence and descending severity: very common (≥ 1/10); frequent (≥ 1/100,
Storage conditions
Keep out of the reach of children. Store at a temperature not exceeding 25 °C. Store in original packaging.
Buy Ketonal capsules 50 mg No. 25 in a pharmacy
Price for Ketonal capsules 50 mg No. 25
Instructions for use for Ketonal capsules 50 mg No. 25
KETONAL
Side effects
According to the World Health Organization (WHO), adverse effects are classified according to their frequency as follows: very common (≥1/10), common (≥1/100, <1/10), uncommon (≥1/1000, < 1/100), rare (≥1/10000, <1/1000) and very rare (<1/10000);
frequency unknown (the frequency of events cannot be determined from the available data). Hematopoietic and lymphatic system disorders
rarely: hemorrhagic anemia; frequency unknown: agranulocytosis, thrombocytopenia, bone marrow dysfunction.
Immune system disorders
frequency unknown: anaphylactic reactions (including anaphylactic shock).
Nervous system disorders
uncommon: headache, dizziness, drowsiness; rarely: paresthesia; frequency unknown: convulsions, disturbance of taste.
Mental disorders
frequency unknown: emotional lability.
Sensory disorders
rarely: blurred vision, tinnitus.
Cardiovascular disorders
frequency unknown: heart failure, increased blood pressure, vasodilation.
Respiratory system disorders
rarely: exacerbation of bronchial asthma; frequency unknown: bronchospasm (especially in patients with hypersensitivity to NSAIDs), rhinitis.
Gastrointestinal disorders
often: nausea, vomiting, dyspepsia, abdominal pain; uncommon: constipation, diarrhea, bloating, gastritis; rarely: peptic ulcer, stomatitis; very rarely: exacerbation of ulcerative colitis or Crohn's disease, gastrointestinal bleeding, perforation.
Disorders of the liver and biliary tract
rarely: hepatitis, increased activity of liver transaminases, increased bilirubin concentration.
Skin and subcutaneous tissue disorders
uncommon: skin rash, itching; frequency unknown: photosensitivity, alopecia, urticaria, angioedema, erythema, bullous rash, including Stevens-Johnson syndrome, toxic epidermal necrolysis.
Renal and urinary tract disorders
frequency unknown: acute renal failure, interstitial nephritis, nephritic syndrome, nephrotic syndrome, abnormal values of renal function tests.
Other
uncommon: swelling; rarely: weight gain; frequency unknown: increased fatigue.