Ketonal injections are a popular medication in the complex treatment of diseases of the musculoskeletal system. The medicine belongs to the group of non-steroidal anti-inflammatory drugs. The solution in ampoules is used for intramuscular and intravenous administration.
Composition and indications
The main active ingredient in the drug is ketoprofen. Ketonal ampoules contain 2 ml of solution with 100 mg of active substance. The injection liquid may be clear or have a slightly yellowish tint.
Ketoprofen has anti-inflammatory, analgesic and antipyretic properties. It is quickly absorbed from the gastrointestinal tract, which causes quick results.
Ketonal injections are indicated in the treatment of various diseases for the symptomatic treatment of disease processes. They are most effective for relieving pain that accompanies inflammatory and degenerative diseases of the musculoskeletal system.
Ketonal injections, the instructions for use confirm this, improve the condition of rheumatoid and seronegative arthritis during exacerbation of diseases when severe pain occurs.
For symptomatic treatment, the drug is also prescribed:
- For gout. With the development of this disease, uric acid salts are deposited in the joints, which causes their modification and severe pain. Ketonal injections are indicated to relieve an attack of gout, which is characterized by swelling in the area of one of the joints and pressing pain in it.
- For osteoarthritis. As a rule, the cause of the pathology is damage to the joint or an inflammatory process in it. Ketonal injections can relieve pain and maintain joint mobility.
Ketonal injections are prescribed to relieve post-traumatic and postoperative pain syndrome. Thanks to its pronounced analgesic properties, the drug improves the condition of cancer, algodisminorrhea, and inflammation of the pelvic organs. It can be used to relieve severe headaches caused by various reasons, as well as in other pathological conditions:
- Migraine.
- Myalgia.
- Bursitis.
- Radiculitis.
- Neuralgia.
Is it possible to take Milgamma, Movalis and Mydocalm at the same time?
If the pain is severe, causes discomfort, cannot be tolerated, and affects the usual rhythm of life, then the doctor may prescribe a combined treatment system. Milgamma and Mydocalm are often prescribed along with Movalis, which have shown good results in the treatment of certain diseases (intervertebral hernia, osteochondrosis). But before injecting Movalis, Milgamma and Mydocalm, you need to visit a doctor, since these drugs in any case have contraindications and the risk of developing negative reactions in the patient’s body.
- Movalis is a non-steroidal anti-inflammatory drug. Its use is indicated in the treatment of diseases of the musculoskeletal system to relieve inflammation, pain and lower temperature.
- Milgamma is a combined multivitamin product consisting of B vitamins. When diagnosing diseases of the musculoskeletal system, Milgamma injections are prescribed to improve blood circulation, nourish cartilage and bone tissue, and strengthen the nervous system.
- Mydocalm is a muscle relaxant. The drug relieves muscle spasms, reduces muscle tension, reduces compression of nerve endings and eliminates pain.
The classic treatment regimen with Movalis, Milgamma and Mydocalm looks like this: during the first three days, Movalis injections are administered once a day, then the patient is transferred to the tablet form of the drug. Milgamma is administered 2 ml IM once a day. Then injections are given two to three times a week, or the tablet form of Milgamma is prescribed.
Mydocalm injections (100 mg) are indicated 2 times a day, the dosage is 100 mg. To enhance the effect, injections can be given on the same day.
Thus, Movalis, Milgamma and Mydocalm are prescribed to eliminate the symptoms of the disease. Despite the fact that the drugs belong to different pharmacological groups, when used together they give positive dynamics during therapy.
It is worth remembering that for patients who are sensitive or intolerant to lidocaine, this treatment regimen is contraindicated.
Contraindications
A contraindication to ketonal injections is hypersensitivity to the active ingredient or other non-steroidal anti-inflammatory drugs, as well as salicylates.
It is prohibited to use ketonal injections in many other cases. All of them are indicated in the instructions for use of the drug. Injections with the drug are not prescribed until the age of 15 years, in the third trimester of pregnancy and during lactation.
Main contraindications of the drug:
- Exacerbation of peptic ulcer of the stomach and duodenum.
- Heart, liver and kidney failure.
- Blood clotting disorders.
You should stop using the drug if you are diagnosed with Crohn's disease and ulcerative colitis, or if you suspect gastrointestinal or other bleeding. Ketonal injections are not prescribed; the instructions warn about this after coronary artery bypass surgery.
Ketonal solution for injection 100 mg/2 ml in ampoules 2 ml No. 10
Name
Description
A transparent solution from colorless to slightly yellowish in color, with virtually no visible inclusions.
Main active ingredient
Release form
2 ml ampoules made of dark glass. 10 ampoules along with an insert in a cardboard box.
Dosage
special instructions
Do not mix tramadol and ketoprofen in the same infusion fluid due to sediment formation. Infusion bottles should be wrapped in black paper or aluminum foil as ketoprofen is light sensitive.
pharmachologic effect
Pharmacodynamics
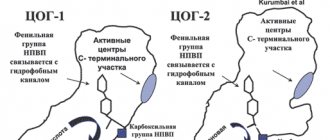
Mechanism of action Ketoprofen inhibits the synthesis of prostaglandins and leukotrienes by blocking the enzyme cyclooxygenase (at least two isoenzymes: cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2)), which catalyzes the synthesis of prostaglandins in the metabolism of arachidonic acid. Ketoprofen stabilizes lysosomal membranes in vitro and in vivo, in high concentrations inhibits the synthesis of leukotrienes in vitro and has anti-bradykinin activity. The mechanism of the antipyretic effect of ketoprofen is unknown. It is possible that ketoprofen inhibits the synthesis of prostaglandins in the central nervous system (most likely in the hypothalamus). In some women, ketoprofen reduces the symptoms of primary dysmenorrhea, probably by suppressing the synthesis and/or effectiveness of prostaglandins.
Pharmacokinetics
Absorption Average plasma levels measured 5 minutes after the start of an intravenous infusion of 100 mg ketoprofen and 4 minutes after the end of the administration were 26.4 ± 5.4 μg/ml. Bioavailability - 90%. In most patients, when administered intramuscularly, ketoprofen was detected in the blood within 15 minutes, and peak plasma concentrations were reached 2 hours after administration. The bioavailability of ketoprofen by injection increases linearly with increasing dose of the drug. Distribution: 99% of ketoprofen is bound to plasma proteins, predominantly albumin. The volume of distribution in tissues is 0.1-0.2 l/kg. Ketoprofen penetrates into the synovial fluid. Three hours after the administration of 100 mg of ketoprofen, its concentration in plasma is about 3 μg/ml, and the concentration in synovial fluid is 1.5 μg/ml. After nine hours, its concentration in plasma is about 0.3 μg/ml, and the concentration in synovial fluid is 0.8 μg/ml. This means that ketoprofen slowly penetrates into the synovial fluid and is also slowly eliminated from it, against the background of a continuing decrease in plasma concentration. Equilibrium concentrations of ketoprofen are established 24 hours after its administration. In elderly patients, steady-state concentration was reached after 8.7 hours and was 6.3 mcg/ml. Already 15 minutes after a single intramuscular injection, ketoprofen was detected in the synovial fluid, and its peak concentration was reached after 2 hours (1.3 μg/ml). Metabolism Ketoprofen is extensively metabolized by hepatic microsomal enzymes. It binds to glucuronic acid and is excreted from the body in this form. After oral administration, its plasma clearance is 1.16 ml/min/kg. Due to its rapid metabolism, its biological half-life is only two hours. Since ketoprofen is metabolized primarily in the liver, liver failure may cause a prolongation of the half-life, in these circumstances the possible accumulation must be taken into account. Excretion Up to 80% of ketoprofen is excreted in the urine, mainly (over 90%) in the form of ketoprofen glucuronide, and about 10% is excreted in the feces. In patients with renal failure, ketoprofen is eliminated more slowly and its biological half-life increases by an hour. Pharmacokinetics in special groups of patients In patients with liver failure, ketoprofen may accumulate in tissues. Its metabolism and elimination are slowed in older people. It is clinically significant only in patients with reduced renal function. Incompatibility Tramadol and ketoprofen should not be mixed in the same bottle due to the formation of sediment. Infusion bottles should be wrapped in black paper or aluminum foil as ketoprofen is light sensitive.
Indications for use
Symptomatic treatment of acute painful episodes occurring during inflammatory diseases of the musculoskeletal system.
Directions for use and doses
For parenteral use. Recommended dose The maximum daily dose is 200 mg. It is recommended not to prescribe injections for longer than 3 days. Upon achieving a satisfactory response, Ketonal is prescribed in oral form. Intramuscular administration One ampoule (100 mg) is administered intramuscularly once or twice a day. The drug can, if necessary, be combined with ketoprofen in the form for oral, rectal or transdermal use. Intravenous administration Ketoprofen infusion should be carried out only in a hospital setting. The infusion lasts for 0.5-1 hour. The infusion can be carried out no more than two days in a row. Short-term intravenous infusion 100 to 200 mg of ketoprofen, diluted in 100 ml of 0.9% sodium chloride solution, is administered over 0.5-1 hour. Continuous intravenous infusion 100 to 200 mg of ketoprofen, diluted in 500 ml of solution for infusion (0.9% sodium chloride solution, Ringer's lactate, glucose), is administered over 8 hours. Ketoprofen can be combined with centrally acting analgesics. It can be mixed in one bottle with morphine: 10-20 mg of morphine and 100 (up to 200) mg of ketoprofen are diluted in 500 ml of 0.9% sodium chloride solution for injection or Ringer's lactate. Undesirable effects can be minimized by taking the drug at the lowest effective dose for the shortest possible time necessary to relieve symptoms. The maximum daily dose of ketoprofen (regardless of the dosage form) is 200 mg. Before starting treatment at a dose of 200 mg of ketoprofen per day, you should carefully weigh the benefits and possible risks. The use of higher doses is not recommended. Elderly patients In older people, adverse reactions are more likely to have severe consequences. It is recommended that treatment in elderly people begin with the lowest effective doses available. Patients with impaired renal function In patients with mild renal impairment (creatinine clearance below 0.33 ml/s (20 ml/min)), the dose of ketoprofen is reduced. Ketoprofen is contraindicated in patients with severe renal impairment. Patients with impaired liver function In patients with chronic liver disease with reduced serum albumin levels, the dose of ketoprofen is reduced. Ketoprofen is contraindicated in patients with severe liver dysfunction. Children The safety and effectiveness of ketoprofen in children have not been studied.
Use during pregnancy and lactation
Pregnancy Inhibition of prostaglandin synthesis may have a negative effect on pregnancy and/or embryo/fetal development. According to epidemiological data, the use of prostaglandin synthesis inhibitors in early pregnancy is associated with an increased risk of miscarriage, heart defects and gastroschisis. The absolute risk of developing congenital cardiovascular defects increased from less than 1% to approximately 1.5%. The risk is believed to increase with increasing dose and duration of treatment. Administration of a prostaglandin synthesis inhibitor in animals led to an increase in the number of miscarriages before and after implantation and to an increase in embryo-fetal mortality. In addition, an increased incidence of various congenital anomalies, including cardiovascular ones, has been described when a prostaglandin synthesis inhibitor is administered to animals during the period of organogenesis. In the first and second trimesters of pregnancy, ketoprofen should not be prescribed unless absolutely necessary. If ketoprofen is used by a woman trying to become pregnant or in the first or second trimesters of pregnancy, the dose should be as low as possible and the duration of treatment as short as possible. In the third trimester of pregnancy, all prostaglandin synthesis inhibitors can have the following effects on the fetus: - cardiopulmonary toxicity (with premature closure of the Botallian duct and the development of pulmonary hypertension); - impaired renal function, up to the development of renal failure with oligohydroamnion; At the end of pregnancy, prostaglandin synthesis inhibitors can affect the mother and newborn in the following ways: - the likelihood of prolonging bleeding time - an antiplatelet effect that can be observed even when using very low doses of the drug; - inhibition of contractile activity of the uterus, leading to a delay in the onset of labor and its prolongation. Therefore, the use of ketoprofen in the third trimester of pregnancy is contraindicated. Breastfeeding There are no data on the penetration of the drug into human milk. It is not recommended to prescribe ketoprofen to nursing mothers.
Precautionary measures
Concomitant use of the drug with other NSAIDs, including selective cyclooxygenase-2 inhibitors, should be avoided. Undesirable effects can be minimized by taking the drug at the lowest effective dose for the shortest possible time necessary to relieve symptoms. Older people are more likely to experience adverse reactions to NSAIDs, especially gastrointestinal bleeding and perforation, which can be fatal. Patients should be warned that this drug may cause drowsiness, dizziness, or seizures and advised not to drive or operate machinery if these symptoms occur. Patients should be warned about the possible occurrence of visual impairment. In this case, you should not drive vehicles or operate machinery. Gastrointestinal bleeding, ulceration, and perforation Gastrointestinal bleeding, ulceration, or perforation, which may be fatal, has been reported for all NSAIDs and may occur at any time during treatment, with or without preexisting symptoms or severe gastrointestinal disease. tract in the anamnesis. Epidemiological data suggest that ketoprofen may be associated with a high risk of severe gastrointestinal toxicity, which is common with some other NSAIDs, especially when taken in high doses. The risk of gastrointestinal bleeding, ulceration or perforation increases with increasing doses of NSAIDs, in patients with a history of peptic ulcer disease, especially complicated by bleeding or perforation, and in the elderly. Treatment of these patients should be started with the lowest dose available. For these patients, as well as for patients concomitantly taking low doses of acetylsalicylic acid or other drugs that increase the risk of gastrointestinal complications, combination therapy with protective drugs (eg, misoprostol or proton pump blockers) should be considered. Patients with a history of gastrointestinal toxicity, especially the elderly, should report any unusual abdominal symptoms (especially gastrointestinal bleeding), especially early in treatment. Particular caution should be exercised when co-administered with drugs that may increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants (eg, warfarin), selective serotonin reuptake inhibitors or antiplatelet agents such as acetylsalicylic acid. If patients experience gastrointestinal bleeding or ulcers during treatment with Ketonal, treatment with the drug should be discontinued. NSAIDs should be prescribed with caution to patients with a history of gastrointestinal diseases (ulcerative colitis, Crohn's disease), as they may experience exacerbations of these diseases. Risk of gastrointestinal bleeding: the relative risk increases in patients with low body weight. If gastrointestinal bleeding or ulceration occurs, treatment should be stopped immediately. Cardiovascular and Cerebrovascular Effects Patients with a history of hypertension and/or mild to moderate congestive heart failure require appropriate monitoring and counseling as fluid retention and edema have been reported with the use of NSAIDs. Clinical studies and epidemiological data suggest that the use of some non-selective NSAIDs (especially at high doses and during long-term treatment) may be associated with a small increased risk of arterial thrombosis (eg, myocardial infarction or stroke). There is insufficient data to exclude such a risk for ketoprofen. Patients suffering from bronchial asthma in combination with chronic rhinitis, chronic sinusitis and/or nasal polyposis are more likely to experience allergic reactions after taking acetylsalicylic acid and/or non-steroidal anti-inflammatory drugs than other patients. Prescribing the drug may cause an attack of bronchial asthma or bronchospasm, especially in patients with an allergy to acetylsalicylic acid or NSAIDs. In patients with heart failure, cirrhosis and nephrotic syndrome, as well as in patients taking diuretics and in patients with chronic renal failure, especially the elderly, renal function should be carefully monitored at the start of treatment. In such patients, the administration of ketoprofen may cause a decrease in renal blood flow due to inhibition of prostaglandin synthesis and lead to decompensation of renal function. In patients with abnormal liver function tests or a history of liver disease, blood transaminase levels should be periodically monitored, especially during prolonged therapy. Treatment should be discontinued if visual disturbances such as blurred vision occur. Prescribed with caution to persons suffering from alcohol dependence. Severe skin reactions (some of them fatal) associated with the use of NSAIDs, such as exfoliative dermatitis, Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported extremely rarely. The greatest risk of developing these reactions is at the beginning of the course of treatment; in most cases, reactions occur in the first month of treatment. Ketonal should be discontinued at the first appearance of a skin rash, lesions on the mucous membranes or other signs of hypersensitivity. With prolonged treatment, it is necessary to monitor the number of blood cells, as well as liver and kidney function. Like all non-steroidal anti-inflammatory drugs, ketoprofen, due to its anti-inflammatory, analgesic or antipyretic effect, can mask signs of the development of infectious diseases, such as elevated body temperature. Before extensive surgical interventions, the drug must be discontinued. Hyperkalemia due to diabetes mellitus or joint therapy with potassium-sparing drugs. In these circumstances, potassium levels need to be checked regularly. For severe pain, ketoprofen can be used in combination with morphine derivatives. In women, non-selective NSAIDs may reduce fertility. Therefore, they are not recommended for use by women trying to become pregnant. In women experiencing difficulty becoming pregnant or being evaluated for infertility, discontinuation of ketoprofen should be considered. Information on excipients Ketonal, solution for injection The drug contains 12.3% (by volume) ethanol (96%). Each 2 ml of solution contains 200 mg of ethanol, which is equivalent to 5 ml of beer or 2 ml of wine per dose of the drug. Harmful to persons with alcohol dependence. The ethanol (alcohol) content should be considered when used in pregnant and breastfeeding women, children and patients at risk, such as those with liver disease or epilepsy. The drug contains benzyl alcohol (E1519) (40 mg/ampoule), so it should not be administered to premature and newborn babies. In children under 3 years of age, it can cause toxic and anaphylactoid reactions. The drug contains sodium in an amount of less than 1 mmol/ampule (23 mg) and is therefore considered sodium-free. Therapy with the injection form of Ketonal should be carried out under close medical supervision. After eliminating the acute pain episode, it is advisable to switch to other forms of ketoprofen, which are less likely to cause serious reactions. Intramuscular administration of Ketonal for long periods is recommended in a hospital and under the supervision of a physician.
Interaction with other drugs
Drug combinations not recommended Other NSAIDs (including selective cyclooxygenase-2 inhibitors) and salicylates in high doses: increased risk of ulcers and bleeding in the gastrointestinal tract. Anticoagulants (heparin and warfarin) and platelet aggregation inhibitors (eg, ticlopidine, clopidogrel): increased risk of bleeding. If co-administration is necessary, close medical supervision is required. Lithium: Risk of increased plasma lithium levels, which can sometimes reach toxic levels due to decreased renal excretion of lithium. If necessary, plasma lithium concentrations should be carefully monitored and the dose of lithium adjusted during and after NSAID treatment. Methotrexate in doses exceeding 15 mg/week Increased risk of hematotoxicity of methotrexate, especially if used in high doses (>15 mg/week), which is likely due to the displacement of methotrexate from protein binding and reduced renal clearance. At least 12 hours must pass between the end or start of treatment with ketoprofen and treatment with methotrexate. Combinations requiring caution Diuretics Patients receiving diuretics, especially those with dehydration, are at increased risk of renal failure due to decreased renal blood flow due to inhibition of prostaglandin synthesis. Such patients should be adequately hydrated before initiating concomitant use of these drugs, and renal function should be monitored at the start of treatment. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists In patients with impaired renal function (eg, dehydrated patients or the elderly), concomitant use of an ACE inhibitor or angiotensin II receptor antagonist and drugs that inhibit cyclooxygenase may cause additional deterioration. renal function, including possible acute renal failure. Methotrexate in doses below 15 mg/week In the first weeks of combination treatment, it is necessary to monitor the detailed blood picture once a week. If there is any impairment of renal function and in elderly patients, monitoring should be carried out more often. Corticosteroids: Increased risk of developing ulcers or bleeding in the gastrointestinal tract. Pentoxifylline Increases the risk of bleeding. More frequent clinical monitoring and more frequent control of bleeding time are necessary. Combinations that need to be considered Antihypertensive drugs (beta blockers, ACE inhibitors, diuretics) Ketoprofen reduces the effect of antihypertensive drugs (inhibition of vasodilatory prostaglandins). Thrombolytics: increased risk of bleeding. Selective serotonin reuptake inhibitors: increased risk of gastrointestinal bleeding. Probenecid: Concomitant use of probenecid may significantly reduce the plasma clearance of ketoprofen. Combinations that should also be taken into account: Cyclosporine, tacrolimus: risk of additive nephrotoxicity, especially in elderly patients. Risk associated with hyperkalemia A number of drugs and entire therapeutic classes of drugs can contribute to the development of hyperkalemia, e.g., potassium salts, potassium-sparing diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, NSAIDs, heparins (low molecular weight or unfractionated), cyclosporine, tacrolimus and trimethoprim. The development of hyperkalemia may depend on the presence of additional factors. The risk is increased when the above-mentioned drugs are used at the same time. Risk associated with antiplatelet effects Interactions may occur with the simultaneous use of certain drugs that prevent platelet aggregation: tirofiban, eptifibaride, abciximab and iloprost. The simultaneous use of several antiplatelet agents increases the risk of bleeding.
Contraindications
Hypersensitivity to ketoprofen or any of the excipients of the drug. The drug is also contraindicated in the following cases: a history of bronchial asthma, urticaria, rhinitis, bronchospasm or allergic reactions after using ketoprofen or similar active substances, such as other non-steroidal anti-inflammatory drugs (NSAIDs) or salicylates (eg acetylsalicylic acid); severe heart failure; treatment of pain in the perioperative period during coronary artery bypass grafting (CABG); active peptic ulcer, as well as a history of gastrointestinal bleeding, ulcer or perforation; gastrointestinal, cerebrovascular or other active bleeding; history of chronic dyspepsia; severe renal impairment; severe liver dysfunction; predisposition to bleeding; last trimester of pregnancy; children; bleeding disorder or current treatment with anticoagulants.
Compound
Every 2 ml of injection solution (1 ampoule) contains 100 mg of ketoprofen. Excipients: propylene glycol, ethanol (12.3 vol%), benzyl alcohol, sodium hydroxide (for pH correction), water for injection.
Overdose
In adults, the main signs of overdose are headache, dizziness, drowsiness, nausea, vomiting, diarrhea and abdominal pain. In severe intoxication, hypotension, respiratory depression and gastrointestinal bleeding are observed. The patient is immediately hospitalized and given symptomatic treatment. A specific antidote is unknown.
Side effect
If severe side effects occur, treatment should be discontinued. Adverse effects are distributed by organ system class and frequency of occurrence. The frequency of adverse effects is classified as follows: very common (≥ 1/10), common (≥ 1/100,
Storage conditions
Keep out of the reach of children. Store at a temperature not exceeding 25°C.
Buy Ketonal solution for injection 100mg/2ml in ampoules 2ml No. 10 in the pharmacy
Price for Ketonal solution for injection 100 mg/2 ml in ampoules 2 ml No. 10
Instructions for use for Ketonal injection solution 100 mg/2 ml in 2 ml ampoules No. 10
Use during pregnancy
According to studies, ketaprofen can have a negative effect on the general course of pregnancy and provoke spontaneous abortion. In addition, when taking the drug there is a risk of developing intrauterine pathologies of the fetus. At the same time, the likelihood of negative consequences for the mother and unborn child increases depending on the dose and duration of use of the drug.
Ketanol injections are prescribed in the first and second trimesters only in cases where the benefits to the woman outweigh the possible risks to the fetus. In this case, the minimum effective dose is indicated, which is taken in a short course.
Absolute contraindications to taking the drug in the third trimester are explained by the fact that ketanol injections can lead to the possible development of weakness of the uterus and other pathological conditions. In addition, even small doses of the drug during this period of pregnancy can increase the duration of bleeding and have a negative effect on the fetus. The use of ketanol injections during lactation is attributed to the fact that studies on whether ketaprofen gets into breast milk and its effect on the child have not been conducted.
How to take Movalis
General advice for the use of any of the dosage forms of Movalis is that the drug is recommended to be used in the minimum effective dose, and the therapeutic course should last as short as possible. Moreover, during treatment, it is recommended to assess the need for taking the drug and the body’s response. During the day, you can take, regardless of the dosage form of the drug Movalis, only 15 mg/day. This is the maximum for the day.
How to take Movalis medicine correctly?
Movalis tablets and suspension are taken orally during meals with a sufficient amount of water.
- osteoarthritis: 7.5 mg/day. If indicated and the severity of the pain syndrome, it is possible to increase the dose to 15 mg/day;
- rheumatoid arthritis, ankylosing spondylitis: 15 mg/day. (possibly reducing the dose by 2 times);
- Children under 12 years of age are prescribed Movalis in the form of a suspension for the treatment of juvenile rheumatoid arthritis. The dose is calculated based on body weight - 0.125 mg/kg (maximum - 7.5 mg per day).
- children 12-18 years old with juvenile rheumatoid arthritis: 0.25 mg/kg, but not more than 15 mg per day.
It is recommended to use the following dosage regimen (amount of active substance/volume of suspension):
- 12 kg: 1.5 mg/1 ml;
- 24 kg: 3 mg/2 ml;
- 36 kg: 4.5 mg/3 ml;
- 48 kg: 6 mg/4 ml;
- From 60 kg: 7.5 mg/5 ml.
Side effects and overdose
Ketonal injections, the instructions for use warn about this, can cause serious negative side reactions of the body from various systems of the human body. You should carefully read the manufacturer's warnings before using the product for symptomatic treatment.
The most common side effects are insomnia, depression, and asthenia. Nervous system disorders may be accompanied by headaches and increased weakness. Also, after taking the medicine, dyspeptic disorders often occur.
An overdose of ketoprofen poses a health risk. Higher doses may cause vomiting and abdominal pain. The drug, used once in large quantities, can lead to respiratory depression, convulsions, and loss of consciousness. There are risks of developing renal dysfunction. If an overdose is confirmed, you urgently need to rinse your stomach and take absorbents. Further symptomatic treatment is recommended.
Ketonal injections, which are affordable, interact with many other medications. Therefore, it is strictly forbidden to use them for self-medication. Dosages are prescribed by the doctor depending on the patient’s condition and in accordance with the recommendations of the instructions for use.
Special instructions for the use of the drug Movalis:
- for patients with an increased risk of adverse reactions (history of gastrointestinal diseases, presence of risk factors for cardiovascular diseases), the recommended initial daily dose is 7.5 mg;
- For patients with severe renal failure on hemodialysis and the elderly, a dose of 7.5 mg/day is recommended, which should not be exceeded;
- patients with insufficient renal function in which Clcr exceeds 25 ml/min., patients with mild/moderate liver failure, as well as clinically stable cirrhosis do not require dose adjustment.
Important! The required dosage and course duration are determined by the attending physician.
Analogs
Analogues of Movalis on the pharmaceutical market are the following drugs: Mirlox, Artrosan, Melox, Meloxicam, Mataren.
As for Movalis injections, the doctor can replace them with medications containing the same active ingredient: Amelotex, Arthrozan, Meloxicam, Melbek, Liberum, Bi-xicam, Movasin, Mesipol.
Analogues of the drug Movalis
Movalis/Meloxicam
The drugs are based on the same active substance, so their therapeutic effect is identical. The only difference is the price.
Movalis/Voltaren
The active substance of Voltaren is diclofenac. Frequent development of side effects is noted. Unlike Voltaren, Movalis has a positive effect on cartilage metabolism. Therefore, both drugs are effective for pain relief, but for osteoarthritis, Movalis is better.
Movalis/Nise
The active substance of Nise is nimesulide. Both drugs are characterized by effectiveness in treating heat, inflammation and pain.
But nimesulide is toxic to the liver, but Movalis is not and does not have a similar effect.
Movalis relieves pain more slowly, but its effect is prolonged, while Nise is good for quickly relieving pain.