Glucophage Long is an effective and safe hypoglycemic drug for long-term use
Diabetes mellitus type 2 (DM 2) is a chronic disease characterized by the development of micro- and macrovascular complications. Their prevention is an important task of modern medicine. The article discusses the key links in the pathogenesis of type 2 diabetes, pathophysiologically based approaches to its treatment, in particular the use of drugs whose action is aimed at improving tissue sensitivity to insulin. The pharmacological characteristics of metformin and the possibility of using the drug in patients with chronic kidney disease and non-alcoholic fatty liver disease are discussed in detail. The main advantages of Glucophage Long are reflected.
Table 1. Relative risk of the need to intensify glucose-lowering therapy depending on the starting drug
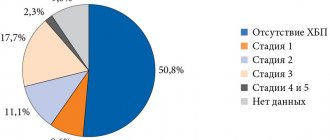
Rice. 1. Prevalence of CKD among patients with type 2 diabetes
Rice. 2. CKD and the risk of developing hypoglycemic conditions (blood glucose < 2.7 mmol/l)
Table 2. Stages of CKD depending on GFR
Table 3. Indexation of CKD by albuminuria level
Rice. 3. Structure of Glucophage Long tablet
Rice. 4. Frequency of gastrointestinal side effects in patients taking metformin and Glucophage Long since the diagnosis of diabetes
Rice. 5. Frequency of gastrointestinal side effects in patients taking metformin and switched to Glucophage Long
Diabetes mellitus type 2 (DM2) is a dangerous, progressive and very common disease. According to the International Diabetes Federation, in 2014 the number of patients in the world reached 387 million, with type 2 diabetes accounting for 90% of cases [1]. The leading reasons for this unfavorable trend are overweight/obesity, physical inactivity and population aging.
Type 2 diabetes is considered a multifactorial disease associated with various concomitant metabolic disorders. It worsens not only the cardiovascular, but also the overall prognosis [2–5]. The main cause of death in patients with type 2 diabetes is cardiovascular disease (CVD).
According to the World Health Organization, the disease increases overall mortality by two to three times [6].
Chronic complications of type 2 diabetes remain the main problem for most patients. Hyperglycemia is a major risk factor for microvascular complications [7]. Macrovascular complications, as a rule, are caused by other significant factors: visceral obesity, insulin resistance (IR), arterial hypertension, dyslipidemia. In combination with hyperglycemia, they significantly worsen the cardiovascular prognosis and require timely intervention [7–10]. Hypoglycemia increases the risk of developing pathology of the heart and blood vessels, which promotes the activation of contrainsular protection [11–13]. Hypoglycemia is known to be an independent risk factor for cardiovascular events. That is why, when choosing a hypoglycemic drug, factors that increase the risk of its development should be taken into account: age, kidney and liver pathology [14–16].
Excess body weight of patients, which often increases during therapy, especially when taking sulfonylureas (SUMs) can also hinder the effective treatment of diabetes [1, 8, 11].
The progress achieved in recent years in the field of diabetology is largely due to the introduction into clinical practice of modern algorithms for the management of patients with diabetes. When choosing a therapeutic agent, not only its effectiveness but also its safety are taken into account [1, 15, 17].
According to the Russian algorithm for specialized medical care for patients with type 2 diabetes, the start and intensification of glucose-lowering therapy is carried out depending on the initial level of glycated hemoglobin (HbA1c). It is recommended to maintain HbA1c levels within selected individual values depending on age, life expectancy, complications and risk of severe hypoglycemia. If pharmacotherapy is insufficiently effective at each stage, it is necessary to change pharmacotherapy no later than six months from the start of treatment [1].
Metformin is recommended as a first-line drug, given its effectiveness in lowering glycemic levels, lack of effect on body weight, low risk of hypoglycemia, good tolerability and relatively low cost [1, 4, 17].
To date, metformin remains the most studied monotherapy drug [18–21]. Metformin is equally effective and safe in both young and elderly patients [13, 18, 20].
In addition, the choice of insulin resistance (IR), a fundamental pathophysiological mechanism for the development of type 2 diabetes, as a therapeutic target made it possible to improve the sensitivity of organs and tissues to insulin. The results of studies have demonstrated the significant role of IR in the development and progression of CVD, as well as in increasing the risk of acute macrovascular complications [3, 4, 22] and unfavorable prognosis [18, 23]. The degree of IR is an independent predictor of kidney disease progression [24].
Metformin has a pronounced inhibitory effect on IR. It should be noted that the elimination of glucose toxicity due to effective reduction of glucose levels also improves tissue sensitivity to insulin [1, 25, 26].
The UKPDS study also noted the ability of metformin to prevent the development of macrovascular complications. Treatment with metformin, compared with PSM and insulin, improved the prognosis of patients to a greater extent: the risk of death from any cause, death from diabetes or heart attack decreased by 36% [21], which was confirmed in subsequent studies [18, 19].
Effects of metformin
The antihyperglycemic effect of metformin is the result of its effect on insulin sensitivity mainly at the level of the liver, as well as muscle and adipose tissue [25–27]. Metformin reduces glucose production mainly due to the suppression of gluconeogenesis: the expression of the gene that induces this process by phosphorylating cyclic adenosine monophosphate (cAMP), a co-activator of CREB protein, is reduced [27, 28]. In addition, the supply of gluconeogenesis substrates to hepatocytes is reduced and enzymes such as pyruvate carboxylase, fructose-1,6-biphosphatase and glucose-6-phosphatase are inhibited.
It is known that excess production of glucose by the liver at night in patients with type 2 diabetes is especially unfavorable due to the stimulation of atherogenesis and the development of resistance to glucose-lowering drugs. Thus, with an increase in fasting blood glucose (FBG) concentration > 6.1 mmol/l, the risk of developing cardiovascular events in the next 12.4 years increases by 1.33 times [29]. Taking metformin helps reduce the level of GKN by 25–30% (on average by 3.3–3.9 mmol/l) [25, 26].
Under the influence of metformin, tissue sensitivity to insulin increases by 18–50%, resulting in increased utilization of glucose by the liver, muscle and fat tissues. In these tissues, metformin promotes the binding of insulin to receptors. There is also an increase in their quantity and affinity, activation of post-receptor mechanisms of insulin action, in particular tyrosine kinase and phosphotyrosine phosphatase [25, 26].
Treatment with metformin also changes the lipid profile: the concentration of triglycerides decreases by 10–20%, low-density lipoprotein cholesterol (LDL) cholesterol by 10%, while the concentration of high-density lipoprotein cholesterol (HDL) increases by 10–20% [20, 25]. Metformin helps reduce the level and rate of oxidation of free fatty acids (by 10–17 and 10–30%, respectively) and activate their re-esterification. As a result, the effects of lipotoxicity are eliminated at all levels, including the liver, adipose and muscle tissue, and the islets of Langerhans [26].
The intestinal effect of metformin is to slow down the rate of absorption of carbohydrates. At the same time, the drug increases the utilization of glucose in the gastrointestinal tract (GIT), enhancing anaerobic glycolysis both in a state of saturation and on an empty stomach. As a result, postprandial glycemia decreases by an average of 20–45% [20, 25]. Thus, metformin makes a significant contribution to the prevention of postprandial peaks in glycemia associated with the risk of premature death from CVD [20].
Prevention of hypoglycemia, given its dangerous consequences, in patients with type 2 diabetes with CVD is extremely important [4, 6, 10]. Thanks to these effects of metformin, glucose levels decrease without the risk of hypoglycemic conditions, which is an undoubted advantage of the drug [20]. The use of metformin leads to a decrease in HbA1c levels by 1.5–2.0% [1, 25].
It is important to note that, without having direct effects on pancreatic beta cells, metformin improves insulin secretion, helping to maintain their functional activity. As IR decreases, the basal level of insulin in the blood serum decreases [20, 25]. In this regard, the results of a retrospective study [17], which analyzed hypoglycemic therapy at the initiation and intensification stages, are interesting. The number of participants was 15,516. The observation period was from 2009 to 2013. Depending on the treatment received, patients were divided into groups: metformin, PSM, thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors. The purpose of the study was to determine the initiation of intensification of therapy (adding another antihyperglycemic agent, including insulin) in patients with type 2 diabetes receiving oral antihyperglycemic drugs (OLDs) for the first time.
Only 57.8% of patients started therapy with metformin. Using Cox regression analysis, it was found that starting therapy with metformin (compared with other DSPs) was associated with a lower need for intensifying therapy in the future (p
Considering that the vast majority of patients with type 2 diabetes are overweight, the primary goal of treatment is to reduce it and maintain it at normal levels [1, 9]. During therapy with metformin, patients experience a decrease in body weight or no increase in weight. In addition, treatment is accompanied by a decrease in visceral fat deposition, which is an independent risk factor for the development of CVD [20, 25]. Recent studies indicate that metformin suppresses the production of the orexigenic peptide ghrelin and increases the level of glucagon-like peptide 1, which has an anorexigenic effect. This partly explains some of the drug's metabolic effects [20, 30].
In recent years, the cardioprotective effects of metformin have been actively discussed [31]. By suppressing increased adhesion of monocytes to the vascular endothelium and lipoidosis, metformin affects the mechanisms of development of atherosclerosis [32, 33]. The drug accelerates the catabolism of LDL, promoting their conversion to HDL, reduces the accumulation of cholesterol esters in the aorta, increases the content of phospholipids and reduces the content of sphingomyelin. Along with this, metformin reduces the proliferation of vascular smooth muscle cells, suppresses the processes of differentiation of monocytes into macrophages, which actively secrete proatherogenic factors. In vitro, metformin inhibited leukocyte-endothelial interaction, as well as endothelial surface expression of intracellular adhesion molecule 1, vascular cell adhesion molecule 1 and E-selectin [25, 26]. The drug has been shown to have a positive effect on the hemostatic system, blood rheology, endothelial function and vascular reactivity [34, 35].
The results of a number of studies have made it possible to reveal other mechanisms underlying the cardioprotective effect of the drug. Thus, in studies by K. Isoda et al. Metformin has been demonstrated to dose-dependently inhibit the release of interleukins (IL) 6 and 8 induced by IL-1-beta in vascular smooth muscle cells, macrophages and endothelial cells [36]. The authors suggest that these processes are based on a decrease in NF-kB translocation.
Since the clinical significance of these properties of metformin has not been conclusively confirmed, their further study is of interest.
Use of metformin in CKD
Diabetic nephropathy is one of the most serious and disabling complications of type 2 diabetes. The risk of developing chronic kidney disease (CKD) in diabetes increases 2.6 times [37, 38]. The disease is detected in approximately one third of patients (Fig. 1).
Diabetic nephropathy is in second place among the causes of death after CVD. It is the main cause of the development of end-stage CKD. In terms of the need for hemodialysis and kidney transplantation, patients with diabetes still hold the lead [14, 16, 39].
Impaired renal function limits the choice of hypoglycemic agent [1, 16, 39] due to the increased risk of hypoglycemia (Fig. 2) due to reduced creatinine clearance, as well as impaired renal gluconeogenesis [2, 16, 39].
The concept of CKD was introduced to unify approaches to the diagnosis, treatment and prevention of kidney damage. It combines various kidney damage and/or decreased function that persists for three months or more, regardless of the primary diagnosis [1, 2]. To make a diagnosis of CKD in the case of preserved or increased glomerular filtration rate (GFR), as well as its slight decrease (60 ≤ GFR
- albuminuria ≥ 30 mg/day or urine albumin/creatinine (al/cr) ratio ≥ 30 mg/g (≥ 3 mg/mol);
- change in urine sediment;
- electrolyte disturbances;
- structural and morphological changes;
- history of kidney transplantation.
With GFR
Assessment of renal dysfunction is necessary not only for the primary diagnosis of kidney pathology, but also for monitoring the effectiveness and safety of therapy, the rate of progression of the pathological process and determining the prognosis. The stage of renal dysfunction is determined by the GFR value, as it most fully reflects the number and total volume of nephron work (Table 2), taking into account the level of albuminuria (Table 3).
The basis for introducing the classification of CKD according to the level of albuminuria was the data that the risk of overall and cardiovascular death and progression of CKD in any range of GFR depends on the rate of albumin excretion (AER) in urine [31].
Of course, the adverse consequences of CKD can be prevented or delayed if diagnosed and treated early [14, 16, 40].
The possibility of using metformin in CKD is currently being actively discussed [40, 41]. It should be noted that metformin is not metabolized in the body and is excreted primarily by the kidneys. With GFR
To prevent lactic acidosis, before prescribing the drug, it is necessary to carefully examine patients in order to identify contraindications to its use. Contraindications include:
- diseases accompanied by tissue hypoxia (heart or pulmonary failure, myocardial infarction, anemia, etc.);
- renal failure or impaired renal function (creatinine clearance
- liver failure, alcoholism;
- pregnancy, lactation;
- acute conditions that may impair renal function (dehydration, acute infection, shock, intravascular administration of radiocontrast agents);
- diabetic ketoacidosis.
Retrospective evaluation of metformin use in University of Chicago patients from 2004–2009. and patients participating in the NHANES study, 1999–2006. demonstrated that metformin use is quite common in GFR
The use of metformin at a GFR of 45–50 ml/min/1.73 m² is safe in the absence of other risk factors for the development of lactic acidosis: poorly controlled diabetes, ketoacidosis, prolonged fasting, excessive alcohol consumption, liver failure and conditions associated with severe hypoxia [1, 42 ].
Use of metformin in NAFLD
In patients with type 2 diabetes, gastrointestinal diseases are often observed, among which non-alcoholic fatty liver disease (NAFLD) is the leading one. The concept of NAFLD combines clinical and morphological changes in the liver - from fatty hepatosis, non-alcoholic steatohepatitis to fibrosis with a possible outcome in the form of cirrhosis, developing due to the influence of various factors in patients who do not drink alcohol in hepatotoxic doses [44, 45]. Since the development of NAFLD is associated with IR, the former is diagnosed in 50–78% of patients with type 2 diabetes. NAFLD, in turn, contributes to the development of CVD [44, 45]. Therefore, the use of metformin in the combination of type 2 diabetes and NAFLD is pathogenetically justified.
As already noted, the main mechanism of action of metformin is realized through the activation of cAMP-dependent protein kinase of the liver, which is accompanied by a decrease in the synthesis of triglycerides from fatty acids and suppression of mitochondrial beta-oxidation, a decrease in the expression of tumor necrosis factor alpha and transcription factors responsible for the synthesis of cholesterol from acetyl-coenzyme A [20, 25, 28].
The results of a retrospective study (2000–2010) including patients with type 2 diabetes with cirrhosis (n = 250) showed that patients receiving metformin (n = 172) at the time of diagnosis of cirrhosis, compared with patients ( n = 78), for whom metformin was discontinued at this stage, the five-year survival rate statistically significantly increased (11.8 versus 5.6 years, p
Glucophage Long
It has been established that 5–10% of patients with diabetes stop taking metformin due to negative events from the gastrointestinal tract [20, 47]. Metformin sustained release, the drug Glucophage Long, can increase the effectiveness of therapy, reduce the frequency of adverse reactions and, as a result, increase patient adherence to treatment [47, 48]. This dosage form appeared thanks to the creation of a tablet with a diffusion system through a gel barrier (Fig. 3). The active substance is contained within a two-layer hydrophilic polymer matrix (inner polymer matrix), surrounded by a closed polymer matrix (outer polymer matrix). After taking the drug, the polymers of the outer dense layer are hydrated and the Glucophage Long tablet turns into a gel-like mass, increasing in size. This transformation helps slow down evacuation through the pylorus and increases the time the drug remains in the stomach. The drug, released for absorption from the inner layer, diffuses through the outer polymer matrix. The release of 90% of the contained drug substance takes about 10 hours, in contrast to the traditional form, when 90% of metformin is released within 30 minutes.
It is important to note that the rate of release of the substance does not depend on intestinal motility or pH level, which minimizes the variability of drug delivery to the gastrointestinal tract.
Pharmacokinetic studies have shown that after a single dose of 2000 mg metformin sustained release, the area under the concentration-time curve was similar to that after a double dose of 1000 mg metformin regular release, indicating the bioequivalence of these dosage forms [48, 49].
It has been proven that the time to reach peak concentration of Glucophage Long increases to 7 hours (for regular release metformin it is 2.5 hours) [49]. Consequently, Glucophage Long has a longer action, which allows it to be taken once a day. This in turn helps to improve adherence and treatment outcomes for type 2 diabetes [47–49].
With similar bioavailability, the peak concentration of sustained-release metformin is reduced by 25% compared to that of regular-release metformin [49].
In a randomized, double-blind study, Glucophage Long demonstrated the same effectiveness in lowering HbA1c levels as regular-release metformin [50].
In addition, thanks to the pharmacokinetics of Glucophage Long, it is possible to avoid a rapid increase in plasma metformin concentrations and, as a consequence, the development of adverse events from the gastrointestinal tract (Fig. 4) [47–50]. Thus, a retrospective analysis of medical records of patients with type 2 diabetes for gastrointestinal tolerability of two forms of metformin showed a significant reduction in the incidence of adverse events from the gastrointestinal tract in patients transferred from therapy with regular-release metformin to sustained-release metformin (Fig. 5) [47, 48] .
Glucophage Long is available in tablets of 500 and 750 mg. The initial dose is 500 mg once a day. The drug is taken during dinner. Its dose, depending on the level of glucose in the blood plasma, can be increased by 500 mg every 10–15 days to a maximum daily dose of four 500 mg tablets or three 750 mg tablets once. If the target glycemic level is not achieved at the maximum daily dose, the possibility of taking the drug twice a day is considered. When switching from Glucophage to Glucophage Long, the initial dose of the latter should be equal to the daily dose of the former.
Conclusion
The choice of PSP requires a balanced approach and assessment of the risk/benefit ratio, especially in patients with risk factors for CVD and CKD. The effectiveness of glucose-lowering therapy can be increased by the use of drugs that affect IR. Metformin has a pronounced inhibitory effect on IR. To date, it remains the most studied drug in terms of effectiveness and safety in the treatment of patients with type 2 diabetes, both in monotherapy and in combination with other PSPs and insulin. Modern prolonged forms of metformin (Glucophage Long) retain all the advantages of traditional metformin, and are also characterized by better tolerability and ease of use, which helps to increase treatment adherence.
Action of the drug
Glucophage tablets quickly relieve the symptoms of hyperglycemia. Their mild action allows you to monitor the course of the disease and timely regulate blood glucose parameters. Along with this effect, the drug has a number of other advantages, the main ones of which doctors consider the prevention of heart, vascular, and kidney diseases.
Opinions of doctors and patients
Speaking about the possibility of drinking alcohol during treatment with Glucophage, doctors clearly state that they cannot be combined. But not all diabetics agree with such a categorical prohibition. Reviews from patients indicate that they do not refuse feasts.
If you plan to drink alcoholic beverages, diabetics do not take another pill. They also prefer to skip taking it the next day. But this can cause the development of short-term decompensated diabetes. The sugar concentration will fluctuate significantly, and alcohol will only worsen the condition. This issue is discussed in more detail further in the article on the effect of alcohol on blood sugar levels.
Imminent danger
You need to understand that even with a single dose of alcohol you can disrupt the functioning of the liver. Drinking alcohol is dangerous for all diabetics, even those who are not yet eligible for drug therapy. When intoxicated with alcohol, severe alcoholic hypoglycemia develops. It appears due to:
- increased insulin secretion, which is stimulated by ethanol;
- blocking the stage of gluconeogenesis, during which lactic acid and alanine are transformed into pyruvic acid;
- depletion of glycogen depot, which should be in the liver.
Therefore, drinking alcohol is always associated with a risk of lactic acidosis. Diabetics should know its main signs:
- apathy;
- muscle pain;
- gagging and other dyspeptic symptoms;
- rapid breathing.
Lack of timely assistance leads to loss of consciousness and subsequent death.
Also, when drinking alcohol and Glucophage, hypoglycemic syndrome may develop. In this condition, glucose levels drop below the minimum acceptable value. The patient experiences the following symptoms:
- weakness;
- headache;
- tremor;
- cardiopalmus;
- numbness of the limbs;
- feeling of extreme hunger;
- visual impairment;
- excitability/inhibition.
Ignoring these symptoms leads to a further decrease in sugar and the possible development of hypoglycemic coma.
The main danger is that while drinking alcohol, you may not notice the symptoms of hypoglycemia or lactic acidosis.
Content:
- Features of the disease
- Alcohol and type 2 diabetes
- Dangerous symptoms of delayed hypoglycemia
- Is it possible to drink alcohol if you have diabetes 4.1 Dry wine 4.2 Vodka 4.3 Beer
- Prohibited types of alcohol
- How to drink correctly if you have non-insulin-dependent diabetes
- When you should absolutely not drink if you have diabetes
The intake of “hot” drinks should always be within reasonable limits.
It is especially important to remember this principle for people who have chronic diseases. Let's try to figure it out: if diabetes is diagnosed, is alcohol allowed or not? Doctors' opinions on this matter differ slightly. Some believe that ethanol should not be used under any circumstances in this condition, while others give patients relief. In fact, everything depends on the unique characteristics of the patient’s body, indicators of his health status, and the therapy used.
How to drink correctly if you have non-insulin-dependent diabetes
Narcologists advise patients to adhere to certain rules that will sometimes allow them to relax with the help of “hot drinks” without causing harm to their health. This means recommendations:
Take alcohol with food, not on an empty stomach. Eat only those foods that are allowed for diabetes.- Drink only high-quality and proven alcohol. You cannot buy cheap wines, formulations with various additives and impurities, preservatives. Poor quality products often cause unpredictable reactions from the body.
- Don't drink before bed. This is associated with an increased risk of delayed hypoglycemia during nighttime sleep.
- Always carry medications with you that help quickly normalize your sugar levels.
- On the day of the feast, take glucose measurements according to the standard scheme, do not forget about their importance.
Following these tips will help minimize possible risks.
Contraindications for use are also general.
- serious disruption of the functioning of internal organs and systems (diabetic precomas, comas, increased acetone levels)
- renal failure
- severe nervous system disorders
- infectious diseases
- respiratory, heart failure, myocardial infarction
- conditions after abdominal surgery and trauma, when the use of insulin is indicated
- before and after radioisotope and x-ray studies (two days before and after manipulations)
- pregnancy, lactation period
- children under 10 years old
Back Next