Pharmacodynamics and pharmacokinetics
Metformin is a biguanide with a hypoglycemic effect that can reduce the concentration of glucose in the blood plasma. However, it does not stimulate the production of insulin , and therefore does not cause hypoglycemia . During treatment, peripheral receptors become more sensitive to insulin, and glucose utilization by cells increases. The synthesis of glucose by the liver is reduced due to inhibition of glycogenolysis and gluconeogenesis. There was a delay in glucose absorption in the gastrointestinal tract.
The active component of the drug stimulates the production of glycogen by acting on glycogen synthase. The transport capacity of any membrane glucose transporters increases.
When treated with metformin, patients maintain body weight or notice a moderate decrease. The substance has a beneficial effect on lipid metabolism: reducing the level of total cholesterol, triglycerides and LDL.
Extended-release tablets are characterized by slow absorption. Therefore, the therapeutic effect lasts for at least 7 hours. The absorption of the drug does not depend on food and does not cause accumulation. Slight binding to plasma proteins is noted. Metabolism occurs without the formation of metabolites. The components are excreted unchanged through the kidneys.
Glucophage Long tab prolong 500 mg N30 (Merck)
Lactic acidosis Lactic acidosis is an extremely rare but serious (high mortality in the absence of immediate treatment) complication that can occur due to the accumulation of metformin. Cases of lactic acidosis in patients receiving metformin have occurred primarily in diabetic patients with advanced renal impairment. Other associated risk factors should be considered, such as poorly controlled diabetes, ketosis, prolonged fasting, excessive alcohol consumption, liver failure and any condition associated with severe hypoxia. This may help reduce the incidence of lactic acidosis. The risk of developing lactic acidosis should be considered if nonspecific signs appear, such as muscle cramps accompanied by dyspepsia, abdominal pain, general weakness and severe malaise. Lactic acidosis is characterized by acidotic shortness of breath, vomiting, abdominal pain, muscle cramps and hypothermia followed by coma. Diagnostic laboratory parameters are a decrease in blood pH (5 mmol/l, increased anion gap and lactate/pyruvate ratio. If lactic acidosis is suspected, stop taking the drug and consult a doctor immediately. Surgeries Metformin use should be discontinued 48 hours before planned surgical operations and can be continued no earlier than 48 hours after, provided that during the examination, renal function was found to be normal. Renal function Since metformin is excreted by the kidneys, before starting treatment, and regularly thereafter, it is necessary to determine CC: at least once a year in patients with normal renal function, and 2-4 times a year in elderly patients, as well as in patients with CK at the lower limit of normal. Particular caution should be exercised in case of possible impairment of renal function in elderly patients, with the simultaneous use of antihypertensive drugs, diuretics or NSAIDs. Other precautions Patients are recommended to continue to follow a diet with even carbohydrate intake throughout the day. Overweight patients are recommended to continue to follow a hypocaloric diet (but not less than 1000 kcal/day). Patients should also exercise regularly. Patients should tell their doctor about any treatment they are undergoing and any infectious diseases, such as respiratory or urinary tract infections. Standard laboratory tests should be performed regularly to monitor diabetes mellitus. Metformin alone does not cause hypoglycemia, however Caution is recommended when used in combination with insulin or other oral hypoglycemic agents (eg, sulfonylureas or repaglinide). Symptoms of hypoglycemia are weakness, headache, dizziness, increased sweating, rapid heartbeat, blurred vision or impaired concentration. The patient must be warned that the inactive components of Glucophage Long may be excreted unchanged through the intestines, which does not affect the therapeutic activity of the drug. Effect on the ability to drive vehicles and operate machinery Monotherapy with Glucophage® Long does not cause hypoglycemia, and therefore does not affect the ability to drive a car or operate machinery. However, patients should be warned about the risk of hypoglycemia when using metformin in combination with other hypoglycemic drugs ( sulfonylurea derivatives, insulin, repaglinide).
Contraindications for use
The drug is not prescribed for:
- sensitivity to metformin and other components;
- diabetic ketoacidosis , precoma , coma ;
- impairment or insufficiency of kidney or liver function;
- acute forms of various diseases;
- extensive injuries and operations;
- chronic alcoholism , alcohol intoxication;
- pregnancy;
- lactic acidosis;
- use 48 hours before or after radioisotope or x-ray studies involving the administration of iodinated contrast agent;
- hypocaloric diets;
- under 18 years of age.
Caution when prescribing this drug should be exercised in relation to elderly patients and people performing heavy physical work, as this can cause the development of lactic acidosis when treating nursing women.
Glucophage® Long
Lactic acidosis
Lactic acidosis is a very rare but serious complication (high mortality in the absence of immediate treatment) that can occur due to the accumulation of metformin. Cases of lactic acidosis when taking metformin occurred mainly in patients with diabetes mellitus with severe renal failure.
Other associated risk factors should be taken into account, such as decompensated diabetes mellitus, ketosis, prolonged fasting, alcoholism, severe infectious disease, liver failure, any condition associated with severe hypoxia and concomitant use of drugs that can cause the development of lactic acidosis (see section " Interaction with other drugs"). This may help reduce the incidence of lactic acidosis. The risk of developing lactic acidosis should be taken into account when nonspecific signs appear, such as muscle cramps accompanied by dyspeptic disorders, abdominal pain and severe asthenia.
Lactic acidosis is characterized by severe malaise with general weakness, acidotic shortness of breath, vomiting, abdominal pain, muscle cramps and hypothermia followed by coma.
Diagnostic laboratory parameters are a decrease in blood pH (less than 7.35), plasma lactate concentration over 5 mmol/l, increased anion gap and lactate/pyruvate ratio. If lactic acidosis is suspected, stop taking the drug and consult a doctor immediately.
Surgical operations
The use of metformin should be discontinued 48 hours before elective surgery and can be continued no earlier than 48 hours after, provided that renal function was found to be normal during the examination.
Kidney function
Since metformin is excreted by the kidneys, before starting treatment and regularly thereafter, it is necessary to determine creatinine clearance: at least once a year in patients with normal renal function, every 3-6 months in patients with creatinine clearance 45-59 ml/min and every 3 months in patients with creatinine clearance 30-44 ml/min.
In case of creatinine clearance less than 30 ml/min, the use of the drug is contraindicated.
Particular caution should be exercised in case of possible impairment of renal function in elderly patients, with dehydration (chronic or severe diarrhea, repeated bouts of vomiting), and with the simultaneous use of antihypertensive drugs, diuretics or non-steroidal anti-inflammatory drugs.
Heart failure
Patients with heart failure have a higher risk of developing hypoxia and renal failure.
Patients with chronic heart failure should have cardiac and renal function monitored regularly while taking metformin. Taking metformin in acute heart failure and chronic heart failure with unstable hemodynamic parameters is contraindicated.
Other Precautions
- Patients are advised to continue to follow a diet with even carbohydrate intake throughout the day. Overweight patients are recommended to continue to follow a hypocaloric diet (but not less than 1000 kcal/day). Patients should also exercise regularly.
— Patients should inform their doctor about any treatment they are undergoing and any infectious diseases such as colds, respiratory tract infections or urinary tract infections.
— It is recommended that routine laboratory tests be performed regularly to monitor diabetes mellitus.
— Metformin, when used alone, does not cause hypoglycemia, but it is recommended to exercise caution when using it in combination with insulin or other oral hypoglycemic agents (for example, sulfonylurea derivatives or repaglinide, etc.). Symptoms of hypoglycemia include weakness, headache, dizziness, increased sweating, rapid heartbeat, blurred vision, or difficulty concentrating.
— It is necessary to warn the patient that the inactive components of the drug Glucophage® Long can be excreted unchanged through the intestines, which does not affect the therapeutic activity of the drug.
Side effects
During drug therapy, the development of lactic acidosis , megaloblastic anemia , and decreased absorption of vitamin B12 is possible.
Disturbances in the functioning of the nervous system and gastrointestinal tract are also possible: changes in taste, nausea, vomiting, pain, diarrhea, loss of appetite. Typically, such symptoms bother you at the beginning of treatment and gradually disappear. To prevent their development, patients are recommended to take metformin together or immediately after meals.
In rare cases, deviations in the activity of the liver and bile and the manifestation of allergic skin reactions .
Instructions for use Glucophage Long (Method and dosage)
The tablets are intended to be taken orally whole with a small volume of liquid. It is recommended to do this daily during dinner.
According to the instructions for use, the choice of dosage of extended-release tablets is carried out individually for a particular patient, taking into account blood glucose concentrations.
Glucophage Long 750 mg and 500 mg can be prescribed as mono- or combination therapy. It is important to strictly adhere to the prescribed dosages and regularly monitor blood sugar levels.
Glucophage Long is an effective and safe hypoglycemic drug for long-term use
Diabetes mellitus type 2 (DM 2) is a chronic disease characterized by the development of micro- and macrovascular complications. Their prevention is an important task of modern medicine. The article discusses the key links in the pathogenesis of type 2 diabetes, pathophysiologically based approaches to its treatment, in particular the use of drugs whose action is aimed at improving tissue sensitivity to insulin. The pharmacological characteristics of metformin and the possibility of using the drug in patients with chronic kidney disease and non-alcoholic fatty liver disease are discussed in detail. The main advantages of Glucophage Long are reflected.
Table 1. Relative risk of the need to intensify glucose-lowering therapy depending on the starting drug
Rice. 1. Prevalence of CKD among patients with type 2 diabetes
Rice. 2. CKD and the risk of developing hypoglycemic conditions (blood glucose < 2.7 mmol/l)
Table 2. Stages of CKD depending on GFR
Table 3. Indexation of CKD by albuminuria level
Rice. 3. Structure of Glucophage Long tablet
Rice. 4. Frequency of gastrointestinal side effects in patients taking metformin and Glucophage Long since the diagnosis of diabetes
Rice. 5. Frequency of gastrointestinal side effects in patients taking metformin and switched to Glucophage Long
Diabetes mellitus type 2 (DM2) is a dangerous, progressive and very common disease. According to the International Diabetes Federation, in 2014 the number of patients in the world reached 387 million, with type 2 diabetes accounting for 90% of cases [1]. The leading reasons for this unfavorable trend are overweight/obesity, physical inactivity and population aging.
Type 2 diabetes is considered a multifactorial disease associated with various concomitant metabolic disorders. It worsens not only the cardiovascular, but also the overall prognosis [2–5]. The main cause of death in patients with type 2 diabetes is cardiovascular disease (CVD).
According to the World Health Organization, the disease increases overall mortality by two to three times [6].
Chronic complications of type 2 diabetes remain the main problem for most patients. Hyperglycemia is a major risk factor for microvascular complications [7]. Macrovascular complications, as a rule, are caused by other significant factors: visceral obesity, insulin resistance (IR), arterial hypertension, dyslipidemia. In combination with hyperglycemia, they significantly worsen the cardiovascular prognosis and require timely intervention [7–10]. Hypoglycemia increases the risk of developing pathology of the heart and blood vessels, which promotes the activation of contrainsular protection [11–13]. Hypoglycemia is known to be an independent risk factor for cardiovascular events. That is why, when choosing a hypoglycemic drug, factors that increase the risk of its development should be taken into account: age, kidney and liver pathology [14–16].
Excess body weight of patients, which often increases during therapy, especially when taking sulfonylureas (SUMs) can also hinder the effective treatment of diabetes [1, 8, 11].
The progress achieved in recent years in the field of diabetology is largely due to the introduction into clinical practice of modern algorithms for the management of patients with diabetes. When choosing a therapeutic agent, not only its effectiveness but also its safety are taken into account [1, 15, 17].
According to the Russian algorithm for specialized medical care for patients with type 2 diabetes, the start and intensification of glucose-lowering therapy is carried out depending on the initial level of glycated hemoglobin (HbA1c). It is recommended to maintain HbA1c levels within selected individual values depending on age, life expectancy, complications and risk of severe hypoglycemia. If pharmacotherapy is insufficiently effective at each stage, it is necessary to change pharmacotherapy no later than six months from the start of treatment [1].
Metformin is recommended as a first-line drug, given its effectiveness in lowering glycemic levels, lack of effect on body weight, low risk of hypoglycemia, good tolerability and relatively low cost [1, 4, 17].
To date, metformin remains the most studied monotherapy drug [18–21]. Metformin is equally effective and safe in both young and elderly patients [13, 18, 20].
In addition, the choice of insulin resistance (IR), a fundamental pathophysiological mechanism for the development of type 2 diabetes, as a therapeutic target made it possible to improve the sensitivity of organs and tissues to insulin. The results of studies have demonstrated the significant role of IR in the development and progression of CVD, as well as in increasing the risk of acute macrovascular complications [3, 4, 22] and unfavorable prognosis [18, 23]. The degree of IR is an independent predictor of kidney disease progression [24].
Metformin has a pronounced inhibitory effect on IR. It should be noted that the elimination of glucose toxicity due to effective reduction of glucose levels also improves tissue sensitivity to insulin [1, 25, 26].
The UKPDS study also noted the ability of metformin to prevent the development of macrovascular complications. Treatment with metformin, compared with PSM and insulin, improved the prognosis of patients to a greater extent: the risk of death from any cause, death from diabetes or heart attack decreased by 36% [21], which was confirmed in subsequent studies [18, 19].
Effects of metformin
The antihyperglycemic effect of metformin is the result of its effect on insulin sensitivity mainly at the level of the liver, as well as muscle and adipose tissue [25–27]. Metformin reduces glucose production mainly due to the suppression of gluconeogenesis: the expression of the gene that induces this process by phosphorylating cyclic adenosine monophosphate (cAMP), a co-activator of CREB protein, is reduced [27, 28]. In addition, the supply of gluconeogenesis substrates to hepatocytes is reduced and enzymes such as pyruvate carboxylase, fructose-1,6-biphosphatase and glucose-6-phosphatase are inhibited.
It is known that excess production of glucose by the liver at night in patients with type 2 diabetes is especially unfavorable due to the stimulation of atherogenesis and the development of resistance to glucose-lowering drugs. Thus, with an increase in fasting blood glucose (FBG) concentration > 6.1 mmol/l, the risk of developing cardiovascular events in the next 12.4 years increases by 1.33 times [29]. Taking metformin helps reduce the level of GKN by 25–30% (on average by 3.3–3.9 mmol/l) [25, 26].
Under the influence of metformin, tissue sensitivity to insulin increases by 18–50%, resulting in increased utilization of glucose by the liver, muscle and fat tissues. In these tissues, metformin promotes the binding of insulin to receptors. There is also an increase in their quantity and affinity, activation of post-receptor mechanisms of insulin action, in particular tyrosine kinase and phosphotyrosine phosphatase [25, 26].
Treatment with metformin also changes the lipid profile: the concentration of triglycerides decreases by 10–20%, low-density lipoprotein cholesterol (LDL) cholesterol by 10%, while the concentration of high-density lipoprotein cholesterol (HDL) increases by 10–20% [20, 25]. Metformin helps reduce the level and rate of oxidation of free fatty acids (by 10–17 and 10–30%, respectively) and activate their re-esterification. As a result, the effects of lipotoxicity are eliminated at all levels, including the liver, adipose and muscle tissue, and the islets of Langerhans [26].
The intestinal effect of metformin is to slow down the rate of absorption of carbohydrates. At the same time, the drug increases the utilization of glucose in the gastrointestinal tract (GIT), enhancing anaerobic glycolysis both in a state of saturation and on an empty stomach. As a result, postprandial glycemia decreases by an average of 20–45% [20, 25]. Thus, metformin makes a significant contribution to the prevention of postprandial peaks in glycemia associated with the risk of premature death from CVD [20].
Prevention of hypoglycemia, given its dangerous consequences, in patients with type 2 diabetes with CVD is extremely important [4, 6, 10]. Thanks to these effects of metformin, glucose levels decrease without the risk of hypoglycemic conditions, which is an undoubted advantage of the drug [20]. The use of metformin leads to a decrease in HbA1c levels by 1.5–2.0% [1, 25].
It is important to note that, without having direct effects on pancreatic beta cells, metformin improves insulin secretion, helping to maintain their functional activity. As IR decreases, the basal level of insulin in the blood serum decreases [20, 25]. In this regard, the results of a retrospective study [17], which analyzed hypoglycemic therapy at the initiation and intensification stages, are interesting. The number of participants was 15,516. The observation period was from 2009 to 2013. Depending on the treatment received, patients were divided into groups: metformin, PSM, thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors. The purpose of the study was to determine the initiation of intensification of therapy (adding another antihyperglycemic agent, including insulin) in patients with type 2 diabetes receiving oral antihyperglycemic drugs (OLDs) for the first time.
Only 57.8% of patients started therapy with metformin. Using Cox regression analysis, it was found that starting therapy with metformin (compared with other DSPs) was associated with a lower need for intensifying therapy in the future (p
Considering that the vast majority of patients with type 2 diabetes are overweight, the primary goal of treatment is to reduce it and maintain it at normal levels [1, 9]. During therapy with metformin, patients experience a decrease in body weight or no increase in weight. In addition, treatment is accompanied by a decrease in visceral fat deposition, which is an independent risk factor for the development of CVD [20, 25]. Recent studies indicate that metformin suppresses the production of the orexigenic peptide ghrelin and increases the level of glucagon-like peptide 1, which has an anorexigenic effect. This partly explains some of the drug's metabolic effects [20, 30].
In recent years, the cardioprotective effects of metformin have been actively discussed [31]. By suppressing increased adhesion of monocytes to the vascular endothelium and lipoidosis, metformin affects the mechanisms of development of atherosclerosis [32, 33]. The drug accelerates the catabolism of LDL, promoting their conversion to HDL, reduces the accumulation of cholesterol esters in the aorta, increases the content of phospholipids and reduces the content of sphingomyelin. Along with this, metformin reduces the proliferation of vascular smooth muscle cells, suppresses the processes of differentiation of monocytes into macrophages, which actively secrete proatherogenic factors. In vitro, metformin inhibited leukocyte-endothelial interaction, as well as endothelial surface expression of intracellular adhesion molecule 1, vascular cell adhesion molecule 1 and E-selectin [25, 26]. The drug has been shown to have a positive effect on the hemostatic system, blood rheology, endothelial function and vascular reactivity [34, 35].
The results of a number of studies have made it possible to reveal other mechanisms underlying the cardioprotective effect of the drug. Thus, in studies by K. Isoda et al. Metformin has been demonstrated to dose-dependently inhibit the release of interleukins (IL) 6 and 8 induced by IL-1-beta in vascular smooth muscle cells, macrophages and endothelial cells [36]. The authors suggest that these processes are based on a decrease in NF-kB translocation.
Since the clinical significance of these properties of metformin has not been conclusively confirmed, their further study is of interest.
Use of metformin in CKD
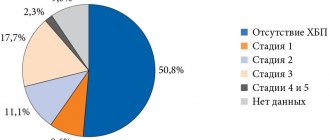
Diabetic nephropathy is one of the most serious and disabling complications of type 2 diabetes. The risk of developing chronic kidney disease (CKD) in diabetes increases 2.6 times [37, 38]. The disease is detected in approximately one third of patients (Fig. 1).
Diabetic nephropathy is in second place among the causes of death after CVD. It is the main cause of the development of end-stage CKD. In terms of the need for hemodialysis and kidney transplantation, patients with diabetes still hold the lead [14, 16, 39].
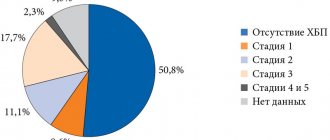
Impaired renal function limits the choice of hypoglycemic agent [1, 16, 39] due to the increased risk of hypoglycemia (Fig. 2) due to reduced creatinine clearance, as well as impaired renal gluconeogenesis [2, 16, 39].
The concept of CKD was introduced to unify approaches to the diagnosis, treatment and prevention of kidney damage. It combines various kidney damage and/or decreased function that persists for three months or more, regardless of the primary diagnosis [1, 2]. To make a diagnosis of CKD in the case of preserved or increased glomerular filtration rate (GFR), as well as its slight decrease (60 ≤ GFR
- albuminuria ≥ 30 mg/day or urine albumin/creatinine (al/cr) ratio ≥ 30 mg/g (≥ 3 mg/mol);
- change in urine sediment;
- electrolyte disturbances;
- structural and morphological changes;
- history of kidney transplantation.
With GFR
Assessment of renal dysfunction is necessary not only for the primary diagnosis of kidney pathology, but also for monitoring the effectiveness and safety of therapy, the rate of progression of the pathological process and determining the prognosis. The stage of renal dysfunction is determined by the GFR value, as it most fully reflects the number and total volume of nephron work (Table 2), taking into account the level of albuminuria (Table 3).
The basis for introducing the classification of CKD according to the level of albuminuria was the data that the risk of overall and cardiovascular death and progression of CKD in any range of GFR depends on the rate of albumin excretion (AER) in urine [31].
Of course, the adverse consequences of CKD can be prevented or delayed if diagnosed and treated early [14, 16, 40].
The possibility of using metformin in CKD is currently being actively discussed [40, 41]. It should be noted that metformin is not metabolized in the body and is excreted primarily by the kidneys. With GFR
To prevent lactic acidosis, before prescribing the drug, it is necessary to carefully examine patients in order to identify contraindications to its use. Contraindications include:
- diseases accompanied by tissue hypoxia (heart or pulmonary failure, myocardial infarction, anemia, etc.);
- renal failure or impaired renal function (creatinine clearance
- liver failure, alcoholism;
- pregnancy, lactation;
- acute conditions that may impair renal function (dehydration, acute infection, shock, intravascular administration of radiocontrast agents);
- diabetic ketoacidosis.
Retrospective evaluation of metformin use in University of Chicago patients from 2004–2009. and patients participating in the NHANES study, 1999–2006. demonstrated that metformin use is quite common in GFR
The use of metformin at a GFR of 45–50 ml/min/1.73 m² is safe in the absence of other risk factors for the development of lactic acidosis: poorly controlled diabetes, ketoacidosis, prolonged fasting, excessive alcohol consumption, liver failure and conditions associated with severe hypoxia [1, 42 ].
Use of metformin in NAFLD
In patients with type 2 diabetes, gastrointestinal diseases are often observed, among which non-alcoholic fatty liver disease (NAFLD) is the leading one. The concept of NAFLD combines clinical and morphological changes in the liver - from fatty hepatosis, non-alcoholic steatohepatitis to fibrosis with a possible outcome in the form of cirrhosis, developing due to the influence of various factors in patients who do not drink alcohol in hepatotoxic doses [44, 45]. Since the development of NAFLD is associated with IR, the former is diagnosed in 50–78% of patients with type 2 diabetes. NAFLD, in turn, contributes to the development of CVD [44, 45]. Therefore, the use of metformin in the combination of type 2 diabetes and NAFLD is pathogenetically justified.
As already noted, the main mechanism of action of metformin is realized through the activation of cAMP-dependent protein kinase of the liver, which is accompanied by a decrease in the synthesis of triglycerides from fatty acids and suppression of mitochondrial beta-oxidation, a decrease in the expression of tumor necrosis factor alpha and transcription factors responsible for the synthesis of cholesterol from acetyl-coenzyme A [20, 25, 28].
The results of a retrospective study (2000–2010) including patients with type 2 diabetes with cirrhosis (n = 250) showed that patients receiving metformin (n = 172) at the time of diagnosis of cirrhosis, compared with patients ( n = 78), for whom metformin was discontinued at this stage, the five-year survival rate statistically significantly increased (11.8 versus 5.6 years, p
Glucophage Long
It has been established that 5–10% of patients with diabetes stop taking metformin due to negative events from the gastrointestinal tract [20, 47]. Metformin sustained release, the drug Glucophage Long, can increase the effectiveness of therapy, reduce the frequency of adverse reactions and, as a result, increase patient adherence to treatment [47, 48]. This dosage form appeared thanks to the creation of a tablet with a diffusion system through a gel barrier (Fig. 3). The active substance is contained within a two-layer hydrophilic polymer matrix (inner polymer matrix), surrounded by a closed polymer matrix (outer polymer matrix). After taking the drug, the polymers of the outer dense layer are hydrated and the Glucophage Long tablet turns into a gel-like mass, increasing in size. This transformation helps slow down evacuation through the pylorus and increases the time the drug remains in the stomach. The drug, released for absorption from the inner layer, diffuses through the outer polymer matrix. The release of 90% of the contained drug substance takes about 10 hours, in contrast to the traditional form, when 90% of metformin is released within 30 minutes.
It is important to note that the rate of release of the substance does not depend on intestinal motility or pH level, which minimizes the variability of drug delivery to the gastrointestinal tract.
Pharmacokinetic studies have shown that after a single dose of 2000 mg metformin sustained release, the area under the concentration-time curve was similar to that after a double dose of 1000 mg metformin regular release, indicating the bioequivalence of these dosage forms [48, 49].
It has been proven that the time to reach peak concentration of Glucophage Long increases to 7 hours (for regular release metformin it is 2.5 hours) [49]. Consequently, Glucophage Long has a longer action, which allows it to be taken once a day. This in turn helps to improve adherence and treatment outcomes for type 2 diabetes [47–49].
With similar bioavailability, the peak concentration of sustained-release metformin is reduced by 25% compared to that of regular-release metformin [49].
In a randomized, double-blind study, Glucophage Long demonstrated the same effectiveness in lowering HbA1c levels as regular-release metformin [50].
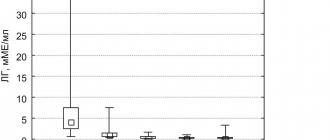
In addition, thanks to the pharmacokinetics of Glucophage Long, it is possible to avoid a rapid increase in plasma metformin concentrations and, as a consequence, the development of adverse events from the gastrointestinal tract (Fig. 4) [47–50]. Thus, a retrospective analysis of medical records of patients with type 2 diabetes for gastrointestinal tolerability of two forms of metformin showed a significant reduction in the incidence of adverse events from the gastrointestinal tract in patients transferred from therapy with regular-release metformin to sustained-release metformin (Fig. 5) [47, 48] .
Glucophage Long is available in tablets of 500 and 750 mg. The initial dose is 500 mg once a day. The drug is taken during dinner. Its dose, depending on the level of glucose in the blood plasma, can be increased by 500 mg every 10–15 days to a maximum daily dose of four 500 mg tablets or three 750 mg tablets once. If the target glycemic level is not achieved at the maximum daily dose, the possibility of taking the drug twice a day is considered. When switching from Glucophage to Glucophage Long, the initial dose of the latter should be equal to the daily dose of the former.
Conclusion
The choice of PSP requires a balanced approach and assessment of the risk/benefit ratio, especially in patients with risk factors for CVD and CKD. The effectiveness of glucose-lowering therapy can be increased by the use of drugs that affect IR. Metformin has a pronounced inhibitory effect on IR. To date, it remains the most studied drug in terms of effectiveness and safety in the treatment of patients with type 2 diabetes, both in monotherapy and in combination with other PSPs and insulin. Modern prolonged forms of metformin (Glucophage Long) retain all the advantages of traditional metformin, and are also characterized by better tolerability and ease of use, which helps to increase treatment adherence.
Overdose
Taking metformin in a dosage of less than 85 g does not cause hypoglycemia . But the possibility of developing lactic acidosis remains.
If symptoms of lactic acidosis appear, you must immediately stop taking the medication and determine the lactate concentration in a hospital setting to clarify the diagnosis. The effectiveness of the procedure for removing lactate and metformin from the body using hemodialysis has been noted. Concomitant symptomatic therapy is also carried out.
Glucophage Long, 500 mg, extended-release tablets, 60 pcs.
Lactic acidosis
Lactic acidosis is a rare but serious (high mortality unless promptly treated) complication that may occur due to accumulation of metformin. Cases of lactic acidosis when taking metformin occurred mainly in diabetic patients with severe renal failure.
Other associated risk factors should be taken into account, such as decompensated diabetes mellitus, ketosis, prolonged fasting, alcoholism, liver failure and any condition associated with severe hypoxia. This may help reduce the incidence of lactic acidosis.
The risk of developing lactic acidosis should be taken into account when nonspecific signs appear, such as muscle cramps accompanied by dyspeptic disorders, abdominal pain and severe asthenia.
Lactic acidosis is characterized by severe malaise with general weakness, acidotic shortness of breath and vomiting, abdominal pain, muscle cramps and hypothermia followed by coma. Diagnostic laboratory parameters are a decrease in blood pH (less than 7.25), plasma lactate concentration over 5 mmol/l, increased anion gap and lactate/pyruvate ratio. If lactic acidosis is suspected, stop taking the drug and consult a doctor immediately.
Surgical operations
The use of metformin should be discontinued 48 hours before elective surgery and can be continued no earlier than 48 hours after, provided that renal function has been found to be normal during the examination.
Kidney function
Since metformin is excreted by the kidneys, before starting treatment and regularly thereafter, it is necessary to determine the content and/or Cl of creatinine in the serum: at least once a year in patients with normal renal function and 2-4 times a year in elderly patients, as well as in patients with Cl creatinine at the lower limit of normal.
In case of creatinine clearance less than 45 ml/min, the use of the drug is contraindicated.
Particular caution should be exercised in case of possible impairment of renal function in elderly patients, with simultaneous use of antihypertensive drugs, diuretics or NSAIDs.
Heart failure
Patients with heart failure have a higher risk of developing hypoxia and renal failure. Patients with CHF should regularly monitor cardiac and renal function while taking metformin. Taking metformin in acute heart failure and CHF with unstable hemodynamic parameters is contraindicated.
Other Precautions
Patients are advised to continue to follow a diet with even carbohydrate intake throughout the day. Overweight patients are recommended to continue to follow a hypocaloric diet (but not less than 1000 kcal/day). Patients should also exercise regularly.
Patients should inform their physician about any treatment they are undergoing and any infectious diseases such as colds, respiratory tract infections, or urinary tract infections.
It is recommended that routine laboratory tests be performed regularly to monitor diabetes mellitus.
Metformin does not cause hypoglycemia when used alone, but caution is recommended when used in combination with insulin or other oral hypoglycemic agents (for example, sulfonylureas or repaglinide, etc.). Symptoms of hypoglycemia include weakness, headache, dizziness, increased sweating, rapid heartbeat, blurred vision, or difficulty concentrating. It is necessary to warn the patient that the inactive components of the drug Glucophage® Long can be excreted unchanged through the intestines, which does not affect the therapeutic activity of the drug.
Impact on the ability to drive vehicles and machinery.
Monotherapy with Glucophage® Long does not cause hypoglycemia and therefore does not affect the ability to drive vehicles and operate machinery.
However, it is possible to develop hypoglycemia when using metformin in combination with other hypoglycemic drugs (sulfonylurea derivatives, insulin, repaglinide, etc.). If symptoms of hypoglycemia appear, you should not drive vehicles or machinery.
Interaction
The development of lactic acidosis can be caused by a combination of the drug with iodine-containing radiocontrast agents. Therefore, 48 hours before and after radiological examination using iodine-containing radiopaque agents, it is recommended to discontinue Glucophage Long.
Concomitant use with drugs with an indirect hyperglycemic effect - hormonal agents or tetracosactide , as well as β2-adrenergic agonists, danazol, Chlorpromazine and diuretics can affect the concentration of glucose in the blood. Therefore, it is necessary to monitor its indicators, and, if necessary, adjust dosages.
In addition, in the presence of renal failure, diuretics contribute to the development of lactic acidosis . Combination with sulfonylurea derivatives , acarbose , insulin , and salicylates often causes hypoglycemia.
Combinations with amiloride , digoxin , morphine , procainamide , quinidine , quinine , ranitidine , triamterene , trimethoprim and vancomycin , which are secreted in the renal tubules, compete with metformin for tubular transport, which increases its concentration.
Glucophage Long tablet prolong 1000 mg x60
Glucophage Long tablet prolong 1000 mg x60, ATX code: A10BA02 (Metformin) Active substance: metformin (metformin) Rec.INN registered by WHO
Dosage form
Glucophage® Long
Tab. with prolonged release 1000 mg: 28, 30, 56 or 60 pcs.reg. No.: LP-002396 from 03/12/14 - Indefinitely Re-registration date: 03/13/19
Release form, packaging and composition of the drug Glucophage® Long
Extended-release tablets are white or off-white, capsule-shaped, biconvex, debossed with “1000” on one side and “MERCK” on the other.
1 tab.
metformin hydrochloride 1000 mg
Excipients: carmellose sodium - 50 mg, hypromellose 2208 - 392.3 mg, magnesium stearate - 7 mg.
Clinical-pharmacological group: Hypoglycemic drug of the biguanide group for oral use Pharmaco-therapeutic group: Hypoglycemic drug for oral use of the biguanide group
pharmachologic effect
Metformin is a biguanide with a hypoglycemic effect, reducing both basal and postprandial plasma glucose levels. It does not stimulate insulin secretion and therefore does not cause hypoglycemia. Increases the sensitivity of peripheral receptors to insulin and the utilization of glucose by cells. Reduces liver glucose production by inhibiting gluconeogenesis and glycogenolysis. Delays the absorption of glucose in the intestine.
Metformin stimulates glycogen synthesis by acting on glycogen synthase. Increases the transport capacity of all types of membrane glucose transporters.
While taking metformin, the patient's body weight either remains stable or decreases moderately.
Metformin has a beneficial effect on lipid metabolism: it reduces the content of total cholesterol, LDL and triglycerides.
Pharmacokinetics
Suction
The average time to reach Cmax of metformin (1214 ng/ml) in blood plasma (TCmax) is 5 hours (range 4-10 hours) after a single oral dose of 1 tablet of Glucophage Long in the dosage form of a 1000 mg extended-release tablet.
At steady state, identical to that of regular-release metformin, Cmax and AUC increase disproportionately to dose. Following a single oral dose of metformin extended-release tablets at a dose of 2000 mg, the AUC is similar to that observed after administration of metformin regular-release tablets at a dose of 1000 mg twice daily.
Intra-individual variability in Cmax and AUC following administration of metformin extended-release tablets is similar to that observed after administration of regular-release tablets.
When metformin extended-release tablets are administered at a dose of 1000 mg after meals, AUC increases by 77% (Cmax and TCmax increase by approximately 1 hour).
The absorption of metformin from extended-release tablets does not change depending on the composition of the food taken.
No accumulation is observed with repeated administration of metformin in the form of extended-release tablets at a dose of up to 2000 mg.
Distribution
Communication with plasma proteins is negligible. Cmax in the blood is lower than Cmax in plasma and is achieved after approximately the same time. Average Vd ranges from 63-276 hp.
Metabolism
No metabolites have been detected in humans.
Removal
Metformin is excreted unchanged by the kidneys. The renal clearance of metformin is >.400 ml/min, indicating that metformin is eliminated by glomerular filtration and tubular secretion. After oral administration, T1/2 is about 6.5 hours.
With impaired renal function, the clearance of metformin decreases in proportion to CC, T1/2 increases, which can lead to an increase in the concentration of metformin in plasma.
Indications for the drug Glucophage® Long
Type 2 diabetes mellitus in adults, especially in obese patients, with ineffective diet therapy and exercise:
as monotherapy, in combination with other oral hypoglycemic agents or with insulin.
Dosage regimen
The drug Glucophage® Long 1000 mg is taken orally. The tablets are swallowed whole, without chewing, with a sufficient amount of liquid, 1 time per day during or after dinner.
The dose of Glucophage® Long 1000 mg is selected by the doctor individually for each patient based on the results of measuring the concentration of glucose in the blood.
Monotherapy and combination therapy in combination with other hypoglycemic agents
Glucophage® Long 1000 mg should be taken once a day during or after dinner. Glucophage Long 1000 mg is prescribed as maintenance therapy for patients taking metformin in the form of regular-release tablets at a dose of 1000 mg or 2000 mg. To switch to Glucophage Long 1000 mg, the daily dose must be equivalent to the daily dose of metformin regular release. Patients taking metformin in the form of regular-release tablets at a dose exceeding 2000 mg are not recommended to switch to Glucophage Long 1000 mg. For patients not taking metformin, the recommended starting dose of Glucophage Long is 500 mg or 750 mg once a day with dinner (the following formulations of Glucophage Long are available: extended-release tablets 500 mg and 750 mg). Every 10-15 days, it is recommended to adjust the dose based on the results of measuring blood glucose concentrations. Slowly increasing the dose promotes better gastrointestinal tolerability. In case of switching from another hypoglycemic agent, dose selection is carried out as described above, starting with the administration of the drug Glucophage® Long 500 mg or 750 mg, with a possible subsequent transition to the drug Glucophage® Long 1000 mg.
Combination with insulin
To achieve better glycemic control, metformin and insulin can be used in combination therapy. The usual starting dose of Glucophage Long is one 500 mg or 750 mg tablet once a day with dinner, while the insulin dose is adjusted based on the results of measuring blood glucose concentrations. Then it is possible to switch to Glucophage® Long 1000 mg.
Daily dose
The maximum recommended dose of Glucophage® Long 1000 mg is 2 tablets per day (2000 mg). If adequate glycemic control cannot be achieved when taking the maximum recommended dose once a day, the maximum dose can be divided into two doses: 1 1000 mg tablet with breakfast and 1 1000 mg tablet with dinner. If adequate glycemic control is not achieved in this case, a switch to regular-release metformin (for example, Glucophage® film-coated tablets) with a maximum daily dose of 3000 mg is possible.
Patients with kidney failure
Metformin can be used in patients with moderate renal failure (creatinine clearance 45–59 ml/min) only in the absence of conditions that may increase the risk of developing lactic acidosis. The initial dose is 500 mg or 750 mg 1 time / day. The maximum dose is 1000 mg/day. Renal function should be carefully monitored every 3-6 months. If CC is below 45 ml/min, the drug should be stopped immediately.
Elderly patients
In elderly patients, the dose of metformin is adjusted based on assessment of renal function, which should be carried out regularly.
Duration of treatment
Glucophage® Long should be taken daily, without interruption. If treatment is stopped, the patient must inform the doctor.
Missing a dose
If a dose is missed, the patient should take the next dose at the usual time. You should not take a double dose of Glucophage® Long.
Side effect
The frequency of side effects of the drug is assessed as follows: very common: ≥1/10 common: ≥1/100, <.1/10 uncommon: ≥1/1000, <.1/100 rare: ≥1/10,000, <.1 /1000 very rare: ≤1/10,000
Metabolic and nutritional disorders
Very rarely - lactic acidosis. With long-term use of metformin, a decrease in the absorption of vitamin B12 may be observed. When megaloblastic anemia is detected, the possibility of such an etiology must be taken into account.
Nervous system disorders
Often - taste disturbance (metallic taste in the mouth).
Gastrointestinal disorders
Very common: nausea, vomiting, diarrhea, abdominal pain and lack of appetite. Most often they occur during the initial period of treatment and in most cases resolve spontaneously. To prevent symptoms, it is recommended to take metformin during or after meals. Slowly increasing the dose may improve gastrointestinal tolerability.
Disorders of the liver and biliary tract
Very rarely - abnormal liver function tests and hepatitis; after discontinuation of metformin, these adverse effects completely disappear.
Skin and subcutaneous tissue disorders
Very rarely - skin reactions such as erythema (redness of the skin), itching, urticaria.
If any of the side effects indicated in the instructions are aggravated, or other side effects not listed in the instructions are noted, the patient should inform the doctor.
Contraindications for use
hypersensitivity to metformin or to any excipient, diabetic ketoacidosis, diabetic precoma, coma, renal failure or impaired renal function (creatinine clearance <.45 ml/min), acute conditions with a risk of developing renal dysfunction, incl. dehydration (with chronic or severe diarrhea, repeated bouts of vomiting), severe infectious diseases (for example, respiratory and urinary tract infections), shock, clinically pronounced manifestations of acute and chronic diseases that can lead to the development of tissue hypoxia (including acute heart failure, chronic heart failure with unstable hemodynamic parameters, respiratory failure, acute myocardial infarction), extensive surgery and trauma when insulin therapy is indicated, liver failure, liver dysfunction, chronic alcoholism, acute alcohol poisoning, lactic acidosis (including . and in the anamnesis), use for a period of less than 48 hours before and within 48 hours after radioisotope or x-ray studies with the introduction of an iodinated contrast agent (for example, intravenous urography), adherence to a hypocaloric diet (less than 1000 kcal/day), pregnancy, children under 18 years of age due to lack of data on use.
Use the drug with caution in patients over 60 years of age who perform heavy physical work, which is associated with an increased risk of developing lactic acidosis, in patients with renal failure (creatinine clearance 45-59 ml/min), and during breastfeeding.
Use during pregnancy and breastfeeding
Decompensated diabetes mellitus during pregnancy is associated with an increased risk of birth defects and perinatal mortality.
Limited evidence suggests that the use of metformin in pregnant women does not increase the risk of birth defects in children.
When planning pregnancy, as well as in the event of pregnancy while using metformin, the drug should be discontinued and insulin therapy should be prescribed. It is necessary to maintain blood glucose concentrations at a level as close to normal as possible to reduce the risk of fetal malformations.
Metformin is excreted in breast milk. No side effects were observed in breastfeeding newborns while taking metformin. However, due to limited data, the use of the drug during breastfeeding is not recommended. The decision to stop breastfeeding should be made taking into account the benefits of breastfeeding and the potential risk of side effects in the baby.
Use for liver dysfunction Contraindication: liver failure, liver dysfunction.
Use for renal impairment
Contraindicated in case of renal failure or impaired renal function (creatinine clearance less than 60 ml/min), in acute conditions with a risk of developing renal dysfunction, incl. dehydration (with chronic or severe diarrhea, repeated bouts of vomiting), severe infectious diseases (for example, respiratory and urinary tract infections), shock.
Use in children
Contraindicated in children and adolescents under 18 years of age due to the lack of data on use.
Use in elderly patients The drug should be used with caution in patients over 60 years of age who perform heavy physical work, which is associated with an increased risk of developing lactic acidosis.
special instructions
Lactic acidosis
Lactic acidosis is an extremely rare but serious complication (high mortality in the absence of immediate treatment) that can occur due to the accumulation of metformin. Cases of lactic acidosis in patients receiving metformin occurred mainly in diabetic patients with severe renal failure.
Other associated risk factors should be taken into account, such as decompensated diabetes mellitus, ketosis, prolonged fasting, alcoholism, liver failure and any condition associated with severe hypoxia. This may help reduce the incidence of lactic acidosis.
The risk of developing lactic acidosis should be taken into account when nonspecific signs appear, such as muscle cramps accompanied by dyspeptic disorders, abdominal pain and severe asthenia.
Lactic acidosis is characterized by severe malaise with general weakness, acidotic shortness of breath, vomiting, abdominal pain, muscle cramps and hypothermia followed by coma. Diagnostic laboratory parameters are a decrease in blood pH (<.7.25), plasma lactate concentration >.5 mmol/l, increased anion gap and lactate/pyruvate ratio. If lactic acidosis is suspected, stop taking the drug and consult a doctor immediately.
Surgical operations
The use of metformin should be discontinued 48 hours before elective surgery and can be continued no earlier than 48 hours after, provided that renal function has been found to be normal during the examination.
Kidney function
Since metformin is excreted by the kidneys, before starting treatment, and regularly thereafter, it is necessary to determine CC: at least once a year in patients with normal renal function, and 2-4 times a year in elderly patients, as well as in patients with CC on lower limit of normal.
Particular caution should be exercised in case of possible impairment of renal function in elderly patients with simultaneous use of antihypertensive drugs, diuretics or NSAIDs.
Heart failure
Patients with heart failure have a higher risk of developing hypoxia and renal failure. Patients with chronic heart failure should have cardiac and renal function monitored regularly while taking metformin.
Taking metformin in acute heart failure and chronic heart failure with unstable hemodynamic parameters is contraindicated.
Other Precautions
Patients are advised to continue to follow a diet with even carbohydrate intake throughout the day. Overweight patients are recommended to continue to follow a hypocaloric diet (but not less than 1000 kcal/day). Patients should also exercise regularly. Patients should inform their physician about any treatment they are undergoing and any infectious diseases such as colds, respiratory tract infections, or urinary tract infections. It is recommended that routine laboratory tests be performed regularly to monitor diabetes mellitus. Metformin does not cause hypoglycemia when used alone, but caution is recommended when used in combination with insulin or other oral hypoglycemic agents (for example, sulfonylureas or repaglinide, etc.). Symptoms of hypoglycemia include weakness, headache, dizziness, increased sweating, rapid heartbeat, blurred vision, or difficulty concentrating.
It is necessary to warn the patient that the inactive components of the drug Glucophage® Long can be excreted unchanged through the intestines, which does not affect the therapeutic activity of the drug.
Impact on the ability to drive vehicles and machinery
Monotherapy with Glucophage® Long does not cause hypoglycemia and therefore does not affect the ability to drive vehicles and machines.
However, it is possible to develop hypoglycemia when using metformin in combination with other hypoglycemic drugs (sulfonylurea derivatives, insulin, repaglinide, etc.). If symptoms of hypoglycemia appear, you should not drive vehicles or machinery.
Overdose
When using metformin at a dose of 85 g (42.5 times the maximum daily dose), the development of hypoglycemia was not observed, but in this case the development of lactic acidosis was observed. Significant overdose or associated risk factors can lead to the development of lactic acidosis.
Treatment: if signs of lactic acidosis appear, treatment with the drug must be stopped immediately, the patient must be urgently hospitalized and, after determining the lactate concentration, the diagnosis must be clarified. The most effective measure for removing lactate and metformin from the body is hemodialysis. Symptomatic treatment is also carried out.
Drug interactions
Contraindicated combinations
Iodine-containing radiocontrast agents: against the background of functional renal failure in patients with diabetes mellitus, radiological examination using iodine-containing X-ray contrast agents can cause the development of lactic acidosis. Glucophage Long should be discontinued depending on renal function 48 hours before or during an X-ray examination using iodine-containing contrast agents and resumed no earlier than 48 hours after, provided that during the examination renal function was found to be normal.
Combinations not recommended
Alcohol. With acute alcohol intoxication, the risk of developing lactic acidosis increases, especially in the case of:
malnutrition, low-calorie diet, liver failure.
While taking the drug, you should avoid drinking alcohol and medications containing ethanol.
Combinations requiring caution
Medicines with indirect hyperglycemic effects (for example, corticosteroids and tetracosactide (systemic and local), beta2-agonists, danazol, chlorpromazine when taken in large doses (100 mg per day) and diuretics): more frequent monitoring of blood glucose concentrations may be required , especially at the beginning of treatment. If necessary, the dose of Glucophage Long can be adjusted during treatment and after its cessation, based on the level of glycemia.
Diuretics: Concomitant use of loop diuretics may lead to the development of lactic acidosis due to possible functional renal failure.
When using the drug Glucophage® Long simultaneously with sulfonylurea derivatives, insulin, acarbose, and salicylates, hypoglycemia may develop.
Nifedipine increases the absorption and Cmax of metformin.
Cationic drugs (amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim and vancomycin) secreted in the renal tubules compete with metformin for tubular transport systems and may lead to an increase in its Cmax.
Colesevelam, when used concomitantly with metformin in the form of extended-release tablets, increases the plasma concentration of metformin (increased AUC without a significant increase in Cmax).
Storage conditions for the drug Glucophage® Long
The drug should be stored out of the reach of children at a temperature not exceeding 30 °C.
Shelf life of the drug Glucophage® Long Shelf life - 3 years.
Terms of sale
The drug is available with a prescription.
Analogs
Level 4 ATX code matches:
Bagomet
Diaformin
Metformin
Formetin
Gliformin
Glucophage
Siofor
The main analogues of this drug:
- Siofor
- Glucophage Long
- Metformin
- Gliformin
- Merifatin
- Formetin
- Diasfor
- Metadiene
- Diaformin
- Rinformin
Reviews about Glucophage
Quite often, patients leave reviews about Glucophage Long 750 mg, since this is the dosage prescribed for the treatment of type 2 diabetes in its middle stage. However, most patients note sufficient effectiveness of the drug. There are often reports that when diabetics with high body weight took this medicine, they subsequently noticed a moderate reduction in weight to more acceptable levels.
As for Glucophage XR 500, the medicine in this dosage can be prescribed at the initial stage of treatment. In the future, a gradual increase in the dose is allowed until the most effective dose is selected.
It should be noted that any hypoglycemic drugs can only be prescribed by a specialist. In addition to competent drug treatment, the doctor will recommend changes in diet and exercise, which should be an integral part of the life of people suffering from diabetes. Only this approach will ensure a normal quality of life and not so acutely feel all the unwanted symptoms of this disorder.
Buy Glucophage Long film-coated tablets 1000 mg No. 60 in pharmacies
Composition 1 tablet contains
Active substance:
metformin hydrochloride - 1000.0 mg;
Excipients:
carmellose sodium - 50.0 mg, hypromellose 2208 - 392.3 mg, magnesium stearate - 7.0 mg. Pharmacological action Pharmacotherapeutic group
Hypoglycemic agent of the biguanide group for oral use. ATX code: A10BA02
pharmachologic effect
Pharmacodynamics
Metformin is a biguanide with a hypoglycemic effect, reducing both basal and postprandial plasma glucose levels. Does not stimulate insulin secretion and therefore does not cause hypoglycemia. Increases the sensitivity of peripheral receptors to insulin and the utilization of glucose by cells. Reduces liver glucose production by inhibiting gluconeogenesis and glycogenolysis. Delays the absorption of glucose in the intestine. Metformin stimulates glycogen synthesis by acting on glycogen synthase. Increases the transport capacity of all types of membrane glucose transporters.
While taking metformin, the patient's body weight either remains stable or decreases moderately. Metformin has a beneficial effect on lipid metabolism: it reduces the content of total cholesterol, low-density lipoproteins and triglycerides.
Pharmacokinetics
Suction
The average time to reach the maximum concentration of metformin (1214 ng/ml) in the blood plasma (TCmax) after a meal is 5 hours (range 4-10 hours) after a single oral dose of 1 tablet of Glucophage® Long in the dosage form of extended-release tablets 1000 mg.
At steady state, identical to that of regular-release metformin, the maximum concentration (Cmax) and area under the concentration-time curve (AUC) increase disproportionately to the dose taken. Following a single oral dose of metformin extended-release tablets 2000 mg, the AUC is similar to that observed after metformin regular-release tablets 1000 mg twice daily.
Intra-individual variability in Cmax and AUC following metformin extended-release tablets is similar to that observed after metformin regular-release tablets.
When metformin extended-release tablets are administered at a dose of 1000 mg after meals, AUC increases by 77% (Cmax increases by 26% and TCmax increases by approximately 1 hour). The absorption of metformin from extended-release tablets is not affected by food intake.
No accumulation is observed with repeated administration of metformin in the form of extended-release tablets at doses up to 2000 mg.
Distribution: Communication with plasma proteins is negligible. Cmax in blood is lower than Cmax in plasma, and is achieved after approximately the same time. The average volume of distribution (Vd) ranges from 63-276 l.
Metabolism No metabolites have been detected in humans.
Excretion Metformin is excreted unchanged by the kidneys. The renal clearance of metformin is >400 ml/min, indicating that metformin is eliminated by glomerular filtration and tubular secretion. After oral administration, the half-life is approximately 6.5 hours.
With impaired renal function, the clearance of metformin decreases in proportion to the clearance of creatinine, the half-life increases, which can lead to an increase in the concentration of metformin in plasma. Indications Type 2 diabetes mellitus in adults, especially in obese patients, with ineffective diet therapy and physical activity:
as monotherapy; in combination with other oral hypoglycemic agents or insulin. Contraindications Hypersensitivity to metformin or any excipient. Diabetic ketoacidosis, diabetic precoma, coma. Renal failure or impaired renal function (creatinine clearance less than 45 ml/min). Acute conditions with a risk of developing renal dysfunction: dehydration (with chronic or severe diarrhea, repeated bouts of vomiting), severe infectious diseases (for example, respiratory tract infections, urinary tract infections), shock. Clinically pronounced manifestations of acute or chronic diseases that can lead to the development of tissue hypoxia (including acute heart failure, chronic heart failure with unstable hemodynamic parameters, respiratory failure, acute myocardial infarction). Major surgical operations and injuries when insulin therapy is indicated (see section “Special Instructions”). Liver failure, liver dysfunction. Chronic alcoholism, acute alcohol poisoning. Pregnancy. Lactic acidosis (including history). Use for less than 48 hours before and within 48 hours after radioisotope or x-ray studies with the introduction of iodine-containing contrast agent (for example, intravenous urography, angiography) (see section “Interaction with other drugs”); Following a hypocaloric diet (less than 1000 kcal/day). Children under 18 years of age due to lack of data on use. Use the drug with caution: in patients over 60 years of age who perform heavy physical work, which is associated with an increased risk of developing lactic acidosis; in patients with renal failure (creatinine clearance 45-59 ml/min) during breastfeeding. Side effects The frequency of side effects of the drug is assessed as follows: Very common: ≥ 1/10 Common: ≥ 1/100, < 1/10 Uncommon: ≥ 1/1000, < 1/100 Rare: ≥ 1/10,000, < 1/ 1000 Very rare: ≤ 1/10,000
Metabolic and nutritional disorders: Very rare: lactic acidosis (see “Special Instructions”). With long-term use of metformin, a decrease in the absorption of vitamin B12 may be observed. When megaloblastic anemia is detected, the possibility of such an etiology must be taken into account.
Nervous system disorders: Common: taste disturbance (metallic taste in the mouth).
Gastrointestinal disorders: Very common: nausea, vomiting, diarrhea, abdominal pain and lack of appetite. Most often they occur during the initial period of treatment and in most cases resolve spontaneously. To prevent symptoms, it is recommended to take metformin during or after meals. Slowly increasing the dose may improve gastrointestinal tolerability.
Liver and biliary tract disorders: Very rare: abnormal liver function tests and hepatitis; after discontinuation of metformin, these adverse effects completely disappear.
Skin and subcutaneous tissue disorders: Very rare: skin reactions such as erythema (redness of the skin), itching, urticaria.
If any of the side effects indicated in the instructions get worse, or other side effects not listed in the instructions are noticed, you should inform your doctor. Interaction Contraindicated combinations
Iodine-containing radiocontrast agents: against the background of functional renal failure in patients with diabetes mellitus, radiological examination using iodine-containing X-ray contrast agents can cause the development of lactic acidosis. Treatment with Glucophage Long should be discontinued depending on renal function 48 hours before or during an X-ray examination using iodine-containing contrast agents and resumed no earlier than 48 hours after, provided that renal function was found to be normal during the examination.
Not recommended combinations
Alcohol: with acute alcohol intoxication, the risk of developing lactic acidosis increases, especially in the case of:
• insufficient nutrition, adherence to a low-calorie diet;
• liver failure.
While taking the drug, you should avoid drinking alcohol and medications containing ethanol.
Combinations requiring caution
Medicines with indirect hyperglycemic effects (for example, glucocorticosteroids (GCS) and tetracosactide (systemic and local), beta2-agonists, danazol, chlorpromazine when taken in large doses (100 mg per day) and diuretics): more frequent monitoring of concentrations may be required blood glucose, especially at the beginning of treatment. If necessary, the dose of Glucophage Long can be adjusted during treatment and after its cessation, based on the level of glycemia.
Diuretics: Concomitant use of loop diuretics may lead to the development of lactic acidosis due to possible functional renal failure.
When using the drug Glucophage® Long simultaneously with sulfonylurea derivatives, insulin, acarbose, and salicylates, hypoglycemia may develop.
Nifedipine increases the absorption and Cmax of metformin. Cationic drugs (amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim and vancomycin) secreted in the renal tubules compete with metformin for tubular transport systems and may lead to an increase in its Cmax.
Colesevelam, when used concomitantly with metformin in the form of extended-release tablets, increases the plasma concentration of metformin (increased AUC without a significant increase in Cmax). How to take, course of administration and dosage Glucophage® Long 1000 mg is taken orally. The tablets are swallowed whole, without chewing, with a sufficient amount of liquid, 1 time per day during or after dinner. The dose of Glucophage® Long 1000 mg is selected by the doctor individually for each patient based on the results of measuring the concentration of glucose in the blood. Monotherapy and combination therapy in combination with other hypoglycemic agents Glucophage® Long 1000 mg should be taken once a day during or after dinner. Glucophage Long 1000 mg is prescribed as maintenance therapy for patients taking metformin in the form of regular-release tablets at a dose of 1000 mg or 2000 mg. To switch to Glucophage Long 1000 mg, the daily dose must be equivalent to the daily dose of metformin regular release. Patients taking metformin in the form of regular-release tablets at a dose exceeding 2000 mg are not recommended to switch to Glucophage Long 1000 mg. For patients not taking metformin, the recommended starting dose of Glucophage Long is 500 mg or 750 mg once daily with dinner (the following formulations of Glucophage Long are available: extended-release tablets 500 mg and 750 mg). Every 10-15 days it is recommended to adjust the dose based on the results of measuring blood glucose concentrations. Slowly increasing the dose promotes better gastrointestinal tolerability. In case of switching from another hypoglycemic agent, dose selection is carried out as described above, starting with the administration of the drug Glucophage® Long 500 mg or 750 mg, with a possible subsequent transition to the drug Glucophage® Long 1000 mg. Combination with insulin To achieve better glycemic control
Metformin and insulin can be used in combination therapy. The usual starting dose of Glucophage Long is one 500 mg or 750 mg tablet once daily with dinner, while the insulin dose is adjusted based on blood glucose measurements. Then it is possible to switch to Glucophage® Long 1000 mg.
Daily dose
The maximum recommended dose of Glucophage® Long 1000 mg is 2 tablets per day (2000 mg). If adequate glycemic control is not achieved when taking the maximum recommended dose once daily, the maximum dose may be divided into two doses: one 1000 mg tablet with breakfast and one 1000 mg tablet with dinner. If adequate glycemic control is not achieved in this case, a switch to regular-release metformin (for example, Glucophage®, film-coated tablets) with a maximum daily dose of 3000 mg is possible.
Patients with kidney failure
Metformin can be used in patients with moderate renal failure (creatinine clearance 45-59 ml/min) only in the absence of conditions that may increase the risk of developing lactic acidosis. The initial dose is 500 mg or 750 mg once daily. The maximum dose is 1000 mg per day. Renal function should be carefully monitored every 3-6 months.
If creatinine clearance is below 45 ml/min, the drug should be discontinued immediately.
Elderly patients
In elderly patients, the dose of metformin is adjusted based on assessment of renal function, which should be carried out regularly (See "Special Instructions").
Duration of treatment
Glucophage® Long should be taken daily, without interruption. If treatment is stopped, the patient must inform the doctor.
Missing a dose
If a dose is missed, the patient should take the next dose at the usual time. You should not take a double dose of Glucophage® Long.
Overdose When using metformin at a dose of 85 g (42.5 times the maximum daily dose), no hypoglycemia was observed. However, in this case, the development of lactic acidosis was observed.
Significant overdose or associated risk factors can lead to the development of lactic acidosis (see “Special Instructions”).
Treatment: if signs of lactic acidosis appear, treatment with the drug must be stopped immediately, the patient must be urgently hospitalized and, after determining the lactate concentration, the diagnosis must be clarified.
The most effective measure for removing lactate and metformin from the body is hemodialysis. Symptomatic treatment is also carried out.
Description Extended-release tablets are white or almost white, capsule-shaped, biconvex, engraved with “1000” on one side and “Merck” on the other.
Special instructions Lactic acidosis Lactic acidosis is a rare but serious complication (high mortality in the absence of urgent treatment) that can occur due to the accumulation of metformin. Cases of lactic acidosis when taking metformin occurred mainly in patients with diabetes mellitus with severe renal failure.
Other associated risk factors should be taken into account, such as decompensated diabetes mellitus, ketosis, prolonged fasting, alcoholism, liver failure and any condition associated with severe hypoxia. This may help reduce the incidence of lactic acidosis.
The risk of developing lactic acidosis should be taken into account when nonspecific signs appear, such as muscle cramps accompanied by dyspeptic disorders, abdominal pain and severe asthenia. Lactic acidosis is characterized by severe malaise with general weakness, acidotic shortness of breath, vomiting, abdominal pain, muscle cramps and hypothermia followed by coma. Diagnostic laboratory parameters are a decrease in blood pH (less than 7.25), plasma lactate concentration over 5 mmol/l, increased anion gap and lactate/pyruvate ratio. If lactic acidosis is suspected, stop taking the drug and consult a doctor immediately.
Surgical operations
The use of metformin should be discontinued 48 hours before elective surgery and can be continued no earlier than 48 hours after, provided that renal function has been found to be normal during the examination.
Kidney function
Since metformin is excreted by the kidneys, before starting treatment and regularly thereafter, it is necessary to determine creatinine clearance: at least once a year in patients with normal renal function, and 2-4 times a year in elderly patients, as well as in patients with creatinine clearance of lower limit of normal.
Particular caution should be exercised in case of possible impairment of renal function in elderly patients, with simultaneous use of antihypertensive drugs, diuretics or non-steroidal anti-inflammatory drugs.
Heart failure
Patients with heart failure have a higher risk of developing hypoxia and renal failure. Patients with chronic heart failure should have cardiac and renal function monitored regularly while taking metformin. Taking metformin in acute heart failure and chronic heart failure with unstable hemodynamic parameters is contraindicated.
Other Precautions Patients are advised to continue to follow a diet with even carbohydrate intake throughout the day. Overweight patients are recommended to continue to follow a hypocaloric diet (but not less than 1000 kcal/day). Patients should also exercise regularly. Patients should inform their physician about any treatment they are undergoing and any infectious diseases such as colds, respiratory tract infections, or urinary tract infections. It is recommended that routine laboratory tests be performed regularly to monitor diabetes mellitus. Metformin does not cause hypoglycemia when used alone, but caution is recommended when used in combination with insulin or other oral hypoglycemic agents (for example, sulfonylureas or repaglinide, etc.). Symptoms of hypoglycemia include weakness, headache, dizziness, increased sweating, rapid heartbeat, blurred vision, or difficulty concentrating. It is necessary to warn the patient that the inactive components of the drug Glucophage® Long can be excreted unchanged through the intestines, which does not affect the therapeutic activity of the drug. Impact on the ability to drive vehicles and machinery
Monotherapy with Glucophage® Long does not cause hypoglycemia and therefore does not affect the ability to drive vehicles and machines.
However, it is possible to develop hypoglycemia when using metformin in combination with other hypoglycemic drugs (sulfonylurea derivatives, insulin, repaglinide, etc.). If symptoms of hypoglycemia appear, you should not drive vehicles or machinery. Storage conditions Store at a temperature not exceeding 30 °C.
Keep out of the reach of children.
Shelf life: 3 years. Active ingredient Metformin Conditions for dispensing from pharmacies By prescription Dosage form tablets
Purpose For adults as prescribed by a doctor
Indications Type 2 diabetes
Price Glucophage Long, where to buy
The price of Glucophage Long 500 mg for 30 pieces per package is about 200 rubles.
- Online pharmacies in RussiaRussia
ZdravCity
- Glucophage long tablets prolong.
750 mg 30 pcs. LLC Nanolek 272 rub. order - Glucophage Long tablets with prolong release. 750 mg 60 pcs. Nanolek LLC
RUR 464 order
- Glucophage long tablets prolong. 500 mg 60 pcs. Merck Sante S.A.S./Nanolek LLC
320 rub. order
- Glucophage Long tab. with prolong. release 1000 mg No. 60 LLC Nanolek
RUB 619 order
- Glucophage Long tablets prolonged action 500 mg 30 pcs. Nanolek LLC
182 RUR order