Codelac Neo syrup: instructions for use
Where can I buy
Registration number : LP-001847
Trade name of the drug : Codelac® Neo
International nonproprietary name: butamirate
Dosage form : syrup.
Composition per 5 ml
Active substance: butamirate citrate – 7.5 mg;
Excipients: sorbitol (Neosorb 70/70 B, sorbitol syrup) 2025.0 mg, glycerol (glycerol) 1450.0 mg, ethanol 95% (ethyl alcohol 95%) 12.69 mg, sodium saccharinate 3.0 mg, benzoin acid 5.75 mg, vanillin 3.0 mg, sodium hydroxide solution 30% 1.55 mg, purified water up to 5 ml.
Description
Colorless liquid with a vanilla odor.
Pharmacotherapeutic group: centrally acting antitussive.
ATX code: R05DB13
Pharmacological properties Pharmacodynamics
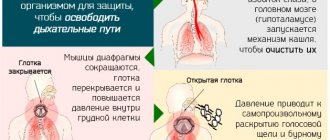
A non-opioid antitussive agent that has a direct effect on the cough center. It has an antitussive, expectorant, moderate bronchodilator and anti-inflammatory effect, improves spirometry (reduces airway resistance) and blood oxygenation.
Pharmacokinetics
Suction
After oral administration, butamirate is quickly and completely absorbed from the gastrointestinal tract. After taking 150 mg of butamirate, the maximum concentration of the main metabolite (2-phenylbutyric acid) in the blood plasma is reached after approximately 1.5 hours and is 6.4 mcg/ml.
Distribution and metabolism
Hydrolysis of butamirate initially to 2-phenylbutyric acid and diethylaminoethoxyethanol begins in the blood. These metabolites also have antitussive activity and, like butamirate, are highly (about 95%) bound to plasma proteins, resulting in their long half-life. 2-phenylbutyric acid is partially metabolized by hydroxylation.
When taking the drug again, no accumulation is observed.
Removal
The half-life of butamirate is 6 hours. Metabolites are excreted mainly by the kidneys. Moreover, 2-phenylbutyric acid is mainly excreted in the form associated with glucuronic acid.
Indications for use
Dry cough of any etiology, including whooping cough; to suppress cough in the preoperative and postoperative period, during surgical interventions and bronchoscopy.
Contraindications
Hypersensitivity to the components of the drug, fructose intolerance, pregnancy (first trimester), breastfeeding period. Children under 3 years of age.
Use of the drug during pregnancy and breastfeeding
There is no data on the safety of the drug during pregnancy and its passage through the placental barrier. The use of the drug in the first trimester of pregnancy is contraindicated. In the second and third trimesters of pregnancy, the use of the drug is possible taking into account the ratio of benefits to the mother and potential risk to the fetus.
The penetration of the drug into breast milk has not been studied, therefore the use of the drug during breastfeeding is not recommended.
Directions for use and doses
The syrup should be taken orally before meals.
The syrup is prescribed to children: aged 3 to 6 years - 5 ml 3 times a day; from 6 to 12 years - 10 ml 3 times a day; 12 years and older - 15 ml 3 times a day. Adults – 15 ml 4 times a day.
When taking the drug, you should use a measuring device.
If the cough persists more than 5 days after starting treatment, you should consult a doctor.
Side effect
From the central nervous system: dizziness, which goes away when the drug is discontinued or the dose is reduced; drowsiness.
From the digestive system: nausea, diarrhea. Allergic reactions: skin rash, itching.
Other: exanthema.
Overdose
Symptoms: nausea, vomiting, drowsiness, diarrhea, dizziness, decreased blood pressure, impaired coordination of movements.
Treatment: activated carbon, saline laxatives, symptomatic therapy (according to indications).
Interaction with other drugs
No drug interactions have been reported for butamirate. During treatment with the drug, it is not recommended to drink alcoholic beverages, as well as drugs that depress the central nervous system (hypnotics, antipsychotics, tranquilizers and other drugs).
special instructions
The syrup contains sodium saccharinate and sorbitol as sweeteners, so it can be used by patients with diabetes.
There is a danger when using the drug in patients with a tendency to develop drug dependence, with liver diseases, alcoholism, epilepsy, and brain diseases.
Impact on the ability to drive a car and operate machinery
It is recommended to refrain from driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions, as the drug can cause dizziness.
Release form
Syrup 1.5 mg/ml.
100 and 200 ml in dark (amber) glass bottles. One bottle, along with instructions for use and a measuring spoon, is placed in a cardboard pack.
Storage conditions
Store at a temperature not exceeding 25 oC. Keep out of the reach of children.
Best before date
2 years. Do not use after the expiration date stated on the packaging.
Conditions for dispensing from pharmacies
Over the counter.
Name and address of the manufacturer/organization receiving claims
OJSC Pharmstandard-Leksredstva, 305022, Russia, Kursk, st. 2nd Aggregatnaya, 1a/18, tel./fax www.pharmstd.ru
Codelac Neo syrup 1.5 mg/ml 200 ml x1
Codelac Neo syrup 1.5 mg/ml 200 ml x1 ATXR05DB13 Butamirate
Compound
Drops for oral administration 20 ml
active substance: ,
butamirate citrate (calculated as 100% substance) 100 mg
excipients: sorbitol (Neosorb 70/70 B, sorbitol syrup), - 8100 mg, glycerol (glycerol), - 5800 mg, ethanol 95% (ethyl alcohol 95%), - 61 mg, sodium saccharinate, - 23 mg, benzoin acid - 23 mg, vanillin - 23 mg, sodium hydroxide solution 30% - 10 mg, purified water - up to 20 ml,
Syrup 5 ml
active substance: ,
butamirate citrate (calculated as 100% substance) 7.5 mg
excipients: sorbitol (Neosorb 70/70, sorbitol syrup) - 2025 mg, glycerol (glycerin) - 1450 mg, ethanol 95% (ethyl alcohol 95%) - 12.69 mg, sodium saccharinate - 3 mg, benzoic acid - 5.75 mg, vanillin - 3 mg, sodium hydroxide solution 30% - 1.55 mg, purified water - up to 5 ml,
Modified-release tablets, film-coated 1 tab.
active substance: ,
butamirate citrate (calculated as 100% substance) 50 mg
excipients: lactose monohydrate (milk sugar) - 241 mg, hypromellose (methocel K4M) - 85 mg, talc - 4 mg, magnesium stearate - 4 mg, colloidal silicon dioxide (Aerosil) - 6 mg, low molecular weight povidone (low molecular weight polyvinylpyrrolidone) - 5 mg,
film shell: Opadry II white 57M280000 (in the form of a powder containing hypromellose (15 cP) - 5.58 mg, titanium dioxide - 4.86 mg, polydextrose - 4.68 mg, talc - 1.26 mg, maltodextrin/dextrin, - 0.9 mg, glycerin/glycerol, - 0.72 mg), - 18 mg,
Description of the dosage form
Drops: colorless to colorless liquid with a yellowish tint, transparent or slightly opalescent, with a vanilla odor.
Syrup: colorless liquid with a vanilla odor.
Tablets: round, biconvex, white film-coated. On a cross section, it is white or almost white.
Pharmacological action Pharmacological action - anti-inflammatory, bronchodilator, expectorant, antitussive.
Pharmacodynamics
A non-opioid antitussive agent that has a direct effect on the cough center. Butamirate citrate is neither chemically nor pharmacologically related to opium alkaloids. It has an antitussive, expectorant, moderate bronchodilator and anti-inflammatory effect, improves spirometry (reduces airway resistance) and blood oxygenation.
Pharmacokinetics
Oral drops, syrup
Suction. After oral administration, the drug is quickly and completely absorbed from the gastrointestinal tract. After taking 150 mg of butamirate, the Cmax of the main metabolite (2-phenylbutyric acid) in the blood plasma is reached after approximately 1.5 hours and is 6.4 mcg/ml.
Distribution and metabolism. Hydrolysis of butamirate, initially to 2-phenylbutyric acid and diethylaminoethoxyethanol, begins in the blood. These metabolites also have antitussive activity, and, like butamirate, are largely (about 95%) bound to plasma proteins, which determines their long half-life. 2-phenylbutyric acid is partially metabolized by hydroxylation.
When taking the drug again, cumulation is not observed.
Excretion. T1/2 is 6 hours. All three metabolites are excreted mainly in the urine. Moreover, 2-phenylbutyric acid is mainly excreted in the form associated with glucuronic acid.
Modified-release film-coated tablets
Absorption is high. After oral administration of a modified-release tablet, the plasma Cmax of the main metabolite (2-phenylbutyric acid) is observed after 9 hours and is 1.4 mcg/ml.
Hydrolysis of butamirate, initially to 2-phenylbutyric acid and diethylaminoethoxyethanol, begins in the blood. These metabolites also have antitussive activity, and, like butamirate, are largely (about 95%) bound to plasma proteins, which determines their long half-life. 2-phenylbutyric acid is partially metabolized by hydroxylation. When taking the drug again, cumulation is not observed.
T1/2 of butamirate - 13 hours. Metabolites are excreted mainly by the kidneys. Moreover, 2-phenylbutyric acid is mainly excreted in the form associated with glucuronic acid.
Indications for Codelac® Neo
dry cough of any etiology (for colds, flu, whooping cough and other conditions),
suppression of cough in the preoperative and postoperative period, during surgical interventions and bronchoscopy.
Contraindications
hypersensitivity to the components of the drug,
pregnancy (first trimester),
breastfeeding period,
fructose intolerance (oral drops, syrup),
lactose intolerance, lactase deficiency, glucose-galactose malabsorption (modified-release film-coated tablets)
children up to 2 months (oral drops), up to 3 years (syrup), up to 18 years (modified-release film-coated tablets).
Use during pregnancy and breastfeeding
There is no data on the safety of the drug during pregnancy and its passage through the placental barrier. The use of the drug in the first trimester of pregnancy is contraindicated. In the second and third trimesters of pregnancy, the use of the drug is possible taking into account the ratio of benefits to the mother and potential risk to the fetus.
The penetration of the drug into breast milk has not been studied, therefore the use of the drug during breastfeeding is not recommended.
Side effects
From the side of the central nervous system: dizziness, which goes away when the drug is discontinued or the dose is reduced, drowsiness.
From the digestive system: nausea, vomiting, diarrhea.
From the skin: exanthema.
Allergic reactions: skin rash, itching.
Interaction
No drug interactions have been reported for butamirate. During treatment with the drug, it is not recommended to consume alcoholic beverages, as well as drugs that depress the central nervous system (including sleeping pills, antipsychotics, tranquilizers).
Directions for use and doses
Inside, before meals.
If the cough persists more than 5 days after starting treatment, you should consult a doctor.
Drops for oral administration
Children from 2 to 12 months - 10 drops 4 times a day, from 1 year to 3 years - 15 drops 4 times a day, over 3 years - 25 drops 4 times a day.
Before using the drug in children under 2 years of age, you should consult your doctor.
Drops for oral administration, 5 mg/ml (1 ml contains 22 drops).
Syrup
Children from 3 to 6 years old - 5 ml 3 times a day, from 6 to 12 years old - 10 ml 3 times a day, 12 years and older - 15 ml 3 times a day, adults - 15 ml 4 times in a day.
When taking the drug, you should use a measuring device.
Modified-release film-coated tablets
Without chewing. 1 table each every 8–12 hours
Overdose
Symptoms: nausea, vomiting, drowsiness, diarrhea, abdominal pain, dizziness, irritability, decreased blood pressure, impaired coordination of movements.
Treatment: administration of activated carbon, gastric lavage, saline laxatives, symptomatic therapy (according to indications).
special instructions
There is a danger when using the drug in patients with a tendency to develop drug dependence, with liver diseases, alcoholism, epilepsy, and brain diseases.
Drops for oral administration, syrup. The drug can be used by patients with diabetes mellitus, because No sucrose or glucose is used as a sweetener.
Modified-release film-coated tablets. Each tablet contains 241 mg of lactose. The drug is contraindicated in patients with lactose intolerance, lactase deficiency, and glucose-galactose malabsorption.
Impact on the ability to drive vehicles and operate machinery. It is recommended to refrain from driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions, because the drug may cause drowsiness and dizziness.
Release form
Drops for oral administration, 5 mg/ml. In a dropper bottle made of dark (amber) glass, 20 ml. 1 bottle in a cardboard pack.
Syrup, 1.5 mg/ml. In a dark (amber) glass bottle, 100 and 200 ml. 1 bottle with a measuring spoon in a cardboard pack.
Modified-release film-coated tablets, 50 mg. In a blister pack made of PVC film and a printed varnished aluminum blank, 10 pcs. In a polymer jar, 30 or 50 pcs. 1, 2 blister packs or a jar in a cardboard pack.
Manufacturer
Drops for oral administration, syrup. OJSC Pharmstandard-Leksredstva. 305022, Kursk, st. 2nd Aggregatnaya, 1a/18.
Tel/fax
Modified-release film-coated tablets. JSC Pharmstandard-UfaVITA. 450077, Russia, Ufa, st. Khudayberdina, 28.
Tel/fax
Conditions for dispensing from pharmacies
Over the counter.
Storage conditions for Codelac® Neo: At a temperature not exceeding 25°C.
Keep out of the reach of children.
The shelf life of Codelac® Neo is 2 years.
Do not use after the expiration date stated on the package.
: R05DB13. Butamirate Pharmacodynamics: Antitussive, has a direct effect on the cough center, has an expectorant, moderate bronchodilator and anti-inflammatory effect, improves spirometry and blood oxygenation.
Pharmacokinetics: Absorption - high. TCmax after oral administration at a dose of 150 mg is 1.5 hours, Cmax is 6.4 mcg/ml. T1/2 - 6 hours. Does not accumulate. Excreted by the kidneys in the form of metabolites.
Indications for use: Dry cough of any etiology: cough in the preoperative and postoperative period, during surgical interventions, bronchoscopy, whooping cough.
Contraindications: Hypersensitivity, pregnancy (1st trimester), lactation period. Children's age: up to 2 months - drops; up to 3 years - syrup; up to 6 years - tablets; up to 12 years - 50 mg tablets.
Dosage regimen: Before surgery, 20 mg is prescribed orally.
Drops: children 6-12 months (8 kg) - 10 drops 4 times a day, 1-3 years (10-15 kg) - 15 drops 4 times a day, over 3 years - 25 drops 4 times a day .
Syrup: children 3-6 years old (15-22 kg) - .5 ml 3 times a day, 6-12 years old (22-30 kg) - 10 ml 3 times a day, over 12 years old (40 kg) - 15 ml, 3 times a day.
Adults - 15 ml, 4 times a day.
Tablets: children 6-12 years old - 5 mg 2 times a day, children over 12 years old - 5 mg 3 times a day, adults - 20 mg 2-3 times a day.
50 mg tablets - for adults, before meals, without chewing, 1 tablet every 8-12 hours.
Side effects: Exanthema, nausea, diarrhea, dizziness, allergic reactions.
Overdose: Symptoms: nausea, vomiting, drowsiness, diarrhea, dizziness, decreased blood pressure. Treatment: activated carbon, saline laxatives, symptomatic therapy (according to indications).
Special instructions: Patients with diabetes mellitus can be prescribed the drug, because Sorbitol and saccharin are used as sweeteners in syrup and drops. 50 mg tablets contain lactose.
Manufacturer: Pharmstandard-Leksredstva OJSC, Russia Registration certificate holder: Pharmstandard-Leksredstva OJSC, Russia Release form: drops for oral administration 5 mg/ml, dropper bottle made of dark (amber) glass Dispensing conditions: without a prescription Registration data: LP- 001808 dated 08/24/2012 Status of the registration certificate: valid (until 2022) Pharmaceutical article number: LP 001808-240812
Manufacturer: Pharmstandard-Leksredstva OJSC, Russia Registration certificate holder: Pharmstandard-Leksredstva OJSC Release form: syrup, 1.5 mg/ml [(bottle) 100/200 ml x 1 + measuring spoon x 1] (cardboard pack) Dispensing conditions: without a prescription Registration data: LP 001847 dated 09/20/2012 Registration certificate status: valid (until 2022)
Manufacturer: Pharmstandard-Leksredstva OJSC, Russia Registration certificate holder: Pharmstandard-Leksredstva OJSC, Russia Release form: syrup 1.5000 mg/ml, dark (amber) glass bottle Dispensing conditions: without a prescription Registration data: LP-001847 dated 09/20/2012 Status of the registration certificate: valid (until 2022) Pharmaceutical article number: LP 001847-200912