Sotalol
Drug interactions
When used simultaneously with class I antiarrhythmic drugs, a pronounced expansion of the QRS complex is possible. The risk of developing ventricular arrhythmia increases.
When used simultaneously with class III antiarrhythmic drugs, a pronounced increase in the duration of the QT interval is possible.
When used simultaneously with calcium channel blockers and antihypertensive drugs, tranquilizers, hypnotics, tricyclic antidepressants, barbiturates, phenothiazines, opioid analgesics, diuretics, vasodilators, a significant decrease in blood pressure is possible.
When used simultaneously with drugs for inhalation anesthesia, the risk of suppression of myocardial function and the development of hypotension increases.
When used simultaneously with amiodarone, arterial hypotension, bradycardia, ventricular fibrillation, and asystole are possible.
With intravenous administration of sotalol while using verapamil and diltiazem, there is a risk of significant deterioration in myocardial contractility and conductivity. An additive inhibitory effect on the sinus and AV nodes is noted.
When used simultaneously with insulins and oral hypoglycemic drugs, especially with increased physical activity, a decrease in blood glucose levels or increased symptoms of hypoglycemia may occur.
When used simultaneously with clonidine, cases of paradoxical arterial hypertension have been described.
In patients receiving beta-blockers, severe arterial hypertension may develop if clonidine is suddenly discontinued. It is believed that this is due to an increase in the content of catecholamines in the circulating blood and an increase in their vasoconstrictor effect.
When used simultaneously with nifedipine, antidepressants, barbiturates, and antihypertensive drugs, the hypotensive effect of sotalol may be enhanced.
When used simultaneously with norepinephrine and MAO inhibitors, severe arterial hypertension is possible.
When used simultaneously with reserpine, methyldopa, guanfacine, cardiac glycosides, severe bradycardia and conduction slowdown may develop.
When used simultaneously with furosemide, indapamide, prenylamine, procainamide, an additive increase in the QT interval is possible.
When used simultaneously with cisapride, the QT interval significantly increases due to an additive effect and the risk of developing ventricular arrhythmia (including pirouette type).
When used simultaneously with erythromycin, the risk of developing ventricular ari increases.
Sotalol Canon (80mg, 160mg)
Antiarrhythmic drugs. Co-administration of sotalol with class IA antiarrhythmic drugs (disopyramide, quinidine, procainamide) and class III drugs (for example, amiodarone) may cause prolongation of the QT interval.
Diuretics that reduce potassium levels. The use of this type of diuretic may lead to hypokalemia or hypomagnesemia, which increases the likelihood of arrhythmias.
Drugs that prolong the QT interval. Sotalol should be used with extreme caution in combination with other drugs that prolong the QT interval, such as class I antiarrhythmics, phenothiazines, tricyclic antidepressants, terfenadine and astemizole, and some quinolone antibiotics.
Blockers of "slow" calcium channels. The simultaneous use of beta-blockers and slow calcium channel blockers can lead to arterial hypotension, bradycardia, conduction disturbances and heart failure. The use of beta-blockers in combination with slow calcium channel blockers that suppress myocardial function (for example, verapamil and diltiazem) should be avoided due to the additive effect of these drugs on atrioventricular conduction and ventricular function.
Drugs that lower catecholamine levels. The simultaneous use of catecholamine depleting agents (for example, reserpine and guanitidine) with beta-blockers leads to an excessive decrease in the tone of the sympathetic nervous system at rest. Patients should be carefully monitored due to possible signs of decreased blood pressure and/or severe bradycardia, which can lead to syncope.
Insulin and hypoglycemic agents for oral administration. Hyperglycemia may develop, in which case it is necessary to adjust the dose of hypoglycemic agents. Sotalol may mask the symptoms of hypoglycemia.
Beta2-adrenergic agonists. When used concomitantly with sotalol, higher doses of beta2-agonists such as salbutamol, terbutaline and isoprenaline may be required.
Digoxin. Taking sotalol does not cause a noticeable effect on the concentration of digoxin in the blood serum. Arrhythmogenic effects were more frequent in patients receiving sotalol concomitantly with digoxin; however, this may be due to chronic heart failure, which is a risk factor for arrhythmogenic effects in patients receiving digoxin.
Clonidine. Beta blockers may potentiate withdrawal hypertension following discontinuation of clonidine; therefore, beta blockers should be discontinued gradually, several days before tapering off clonidine.
Floctafenine. In case of shock or arterial hypotension caused by floctafenine, beta-blockers cause a decrease in compensatory cardiovascular reactions.
Tubocurarine. The use of inhalation anesthesia, including tubocurarine, while taking sotalol increases the risk of myocardial function depression and the development of arterial hypotension.
Amphotericin B and glucocorticosteroids. If used concomitantly, monitor potassium levels.
Laxatives. Concomitant use with laxatives is not recommended.
Sotalol
When used simultaneously with class I antiarrhythmic drugs, a pronounced expansion of the QRS complex is possible. The risk of developing ventricular arrhythmia increases.
When used simultaneously with class III antiarrhythmic drugs, a pronounced increase in the duration of the QT interval is possible.
When used simultaneously with calcium channel blockers and antihypertensive drugs, tranquilizers, hypnotics, tricyclic antidepressants, barbiturates, phenothiazines, opioid analgesics, diuretics, vasodilators, a significant decrease in blood pressure is possible.
When used simultaneously with drugs for inhalation anesthesia, the risk of suppression of myocardial function and the development of hypotension increases.
When used simultaneously with amiodarone, arterial hypotension, bradycardia, ventricular fibrillation, and asystole are possible.
When administered intravenously while using verapamil and diltiazem, there is a risk of significant deterioration in myocardial contractility and conductivity. An additive inhibitory effect on the sinus and AV nodes is noted.
When used simultaneously with insulins and oral hypoglycemic drugs, especially with increased physical activity, a decrease in blood glucose levels or increased symptoms of hypoglycemia may occur.
When used simultaneously with clonidine, cases of paradoxical arterial hypertension have been described.
In patients receiving beta-blockers, severe arterial hypertension may develop if clonidine is suddenly discontinued. It is believed that this is due to an increase in the content of catecholamines in the circulating blood and an increase in their vasoconstrictor effect.
When used simultaneously with nifedipine, antidepressants, barbiturates, and antihypertensive drugs, the hypotensive effect of sotalol may be enhanced.
When used simultaneously with norepinephrine and MAO inhibitors, severe arterial hypertension is possible.
When used simultaneously with reserpine, methyldopa, guanfacine, cardiac glycosides, severe bradycardia and conduction slowdown may develop.
When used simultaneously with furosemide, indapamide, prenylamine, procainamide, an additive increase in the QT interval is possible.
When used simultaneously with cisapride, the QT interval significantly increases due to an additive effect and the risk of developing ventricular arrhythmia (including pirouette type).
When used simultaneously with erythromycin, the risk of developing ventricular ari increases.
Sotalol Canon
Treatment with Sotalol Canon is carried out under the control of heart rate, blood pressure, and ECG. The patient should be taught how to calculate heart rate and instructed about the need for medical consultation if the heart rate is less than 50 beats/min; In smokers, the effectiveness of β-blockers is lower; Patients using contact lenses should take into account that during treatment there may be a decrease in the production of tear fluid.
Withdrawal of the drug
Increased sensitivity to catecholamines is observed in patients after discontinuation of β-blockers. After abrupt cessation of therapy, isolated cases of exacerbation of angina pectoris, the occurrence of arrhythmia, and, in some cases, the development of myocardial infarction have been reported. Therefore, careful monitoring of the patient is recommended, especially with coronary heart disease, if it is necessary to abruptly discontinue long-term therapy with Sotalol Canon. If possible, the dose should be reduced gradually over one or two weeks.
If necessary, it is recommended to initiate replacement therapy. Abrupt cessation of drug use can provoke “hidden” coronary insufficiency, as well as the development of arterial hypertension.
Proarrhythmia
The most dangerous side effect of antiarrhythmic drugs is the exacerbation of existing arrhythmias or the provocation of new arrhythmias. Drugs that prolong the QT interval can cause torsade de pointes (TdP), or torsade de pointes (TdP).
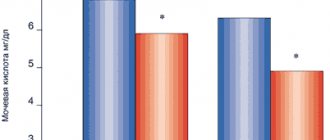
The occurrence of such arrhythmias is associated with a prolongation of the QT interval, a decrease in heart rate, a decrease in serum potassium and magnesium, with high plasma concentrations of sotalol, as well as the simultaneous use of other drugs that prolong the QT interval.
In women, these complications occur more often.
Torsade de pointes tachycardia usually occurs early after the start of therapy or when the dose is increased, and stops spontaneously in most patients. At the same time, dose titration reduces the risk of proarrhythmia.
Rarely, torsade de pointes tachycardia can progress to ventricular fibrillation. Other risk factors for torsade de pointes include significant prolongation of the QT interval with cardiomegaly or congestive heart failure.
Patients with sustained ventricular tachycardia and congestive heart failure have the highest risk of serious proarrhythmias (7%).
The drug Sotalol Canon should be used with extreme caution when the QT interval lasts more than 480 msec, or the dose of the drug must be reduced; therapy should be discontinued when the QT interval exceeds 550 msec.
Electrolyte disturbances
The drug Sotalol Canon should not be used in patients with uncorrected hypokalemia or hyiomagnesemia, because Possible prolongation of the QT interval, as well as an increase in the potential for tachycardia of the “pirouette” type. Particular attention should be paid to the water-electrolyte ratio and acid-base balance in patients with prolonged diarrhea or patients receiving magnesium and/or drugs that remove potassium from the body (diuretics).
Congestive heart failure
Beta-adrenergic receptor blockade may further reduce myocardial contractility and cause progression of heart failure symptoms.
Therapy with Sotalol Canon should be started with caution and at a low dose in patients with impaired contractility of the left ventricular myocardium, controlled by therapy with ACE inhibitors, diuretics, cardiac glycosides, etc.
Myocardial infarction
The positive benefit/risk ratio of using sotalol in patients after a myocardial infarction with impaired left ventricular function has been proven. Careful patient monitoring and dose titration are critical during initiation and continuation of therapy.
Sotalol Canon should not be used in patients with a left ventricular ejection fraction <40% without serious ventricular arrhythmias.
ECG changes
Excessive prolongation of the QT interval, greater than 550 msec, may be a sign of drug toxicity.
Anaphylactoid reactions
In patients using β-blockers with a history of anaphylactic reactions to various allergens, more serious allergic reactions may occur upon repeated contact with the antigen. Such patients may not respond to the usual doses of epinephrine used to treat an allergic reaction.
Diabetes
The drug Sotalol Canon should be used with caution in patients with diabetes mellitus or with a history of episodes of spontaneous hypoglycemia, since the use of beta-blockers may mask signs of the onset of acute hypoglycemia, for example, tachycardia.
Thyrotoxicosis
The use of beta-blockers may mask some clinical signs of hyperthyroidism (eg, tachycardia). Patients with suspected thyrotoxicosis should be carefully monitored to avoid the development of thyrotoxicosis, including thyroid storm, when the drug is discontinued.
Renal dysfunction
Since sotalol is primarily excreted by the kidneys, dose adjustment is required in patients with impaired renal function.
Psoriasis
When using adrenoblockers, the symptoms of psoriasis may worsen.
Sotalol Canon, 20 pcs., 80 mg, tablets
Concomitant administration of sotalol with class IA antiarrhythmic drugs (disopyramide, quinidine, procainamide) and class III drugs (for example, amiodarone) may cause prolongation of the QT interval.
The use of potassium-depleting diuretics may lead to hypokalemia or hypomagnesemia, which increases the likelihood of ari.
Sotalol should be used with extreme caution in combination with other drugs that prolong the QT interval, such as class I antiarrhythmics, phenothiazines, tricyclic antidepressants, terfenadine and astemizole, and some quinolone antibiotics.
The simultaneous use of beta-blockers and slow calcium channel blockers can lead to arterial hypotension, bradycardia, conduction disturbances and heart failure. The use of beta-blockers in combination with slow calcium channel blockers that suppress myocardial function (for example, verapamil and diltiazem) should be avoided due to the additive effect of these drugs on AV conduction and ventricular function.
The simultaneous use of catecholamine depleting agents (for example, reserpine and guanitidine) with beta-blockers leads to an excessive decrease in the tone of the sympathetic nervous system at rest. Patients should be carefully monitored due to possible signs of decreased blood pressure and/or severe bradycardia, which can lead to syncope.
When used simultaneously with insulin and oral hypoglycemic agents, hyperglycemia may develop. In this case, it is necessary to adjust the dose of hypoglycemic agents. Sotalol may mask the symptoms of hypoglycemia.
When used concomitantly with sotalol, it may be necessary to prescribe beta2-agonists such as salbutamol, terbutaline and isoprenaline in higher doses.
The use of sotalol does not cause a noticeable effect on the concentration of digoxin in the blood serum. Arrhythmogenic effects were observed more often in patients receiving sotalol simultaneously with digoxin; however, this may be due to chronic heart failure, which is a risk factor for arrhythmogenic effects in patients receiving digoxin.
Beta blockers may potentiate withdrawal hypertension following discontinuation of clonidine. Therefore, beta blockers should be discontinued gradually, several days before tapering off clonidine.
In case of shock or arterial hypotension caused by floctafenine, beta-blockers cause a decrease in compensatory cardiovascular reactions.
The use of inhalation anesthesia, incl. tubocurarine, while taking sotalol increases the risk of suppression of myocardial function and the development of arterial hypotension.
When used simultaneously with amphotericin B and GCS, potassium concentrations must be monitored.
Concomitant use with laxatives is not recommended.