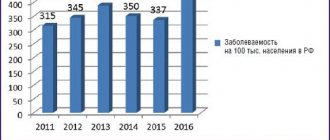
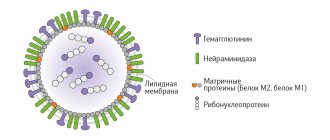
Contraindications and indications for Amoxicillin therapy
The instructions indicate diseases for which antibiotic treatment is recommended:
- pathologies of an inflammatory and infectious nature: bronchitis, tonsillitis, inflammation of the lungs, bladder, urethral canal, lesions of the digestive tract, gynecological diseases, gonorrhea, skin infections;
- exacerbation of chronic gastroduodenitis, gastrointestinal ulcers - for diseases associated with Helicobacter pylori;
- listeriosis, bacillary dysentery, water fever;
- ixodid tick-borne borreliosis, inflammation of the membranes of the spinal cord and brain, septic conditions;
- salmonellosis and carriage, endocarditis (preventive therapy).
Amoxicillin is contraindicated for patients:
- with gastrointestinal diseases accompanied by attacks of vomiting and diarrhea;
- infectious mononucleosis, lymphocytic leukemia, allergic form of diathesis;
- ARVI, hay fever, bronchial asthma;
- individual intolerance to the component composition.
Amoxicillin is not used in combination with metronidazole for pathologies:
- hematopoietic systems;
- mononucleosis, malignant lesion of the hematopoietic department of infectious etiology;
- intolerance to 5-nitroimidazoles;
- diseases of the central nervous system.
Jaundice and liver dysfunction are contraindications for combining the drug with clavulanic acid.
Adverse reactions from treatment with Amoxicillin
Therapeutic manipulations cause the occurrence of:
- nettle fever, erythema, Quincke's edema;
- runny nose, conjunctivitis, joint pain;
- anaphylactic shock, febrile conditions;
- superinfections – with a reduced level of resistance or chronic diseases;
- attacks of dizziness, depression, convulsive syndrome;
- ataxia, peripheral neuropathies, anorexia;
- constipation, nausea, diarrhea, stomatitis;
- glossitis, discomfort in the epigastric region;
- hepatitis, pseudomembranous colitis, interstitial nephritis;
- erythema multiforme, epidermal toxic necrolysis, etc.
The instructions indicate that accidental overdoses of Amoxicillin do not provoke severe intoxication, even with large doses of the drug. Standard adverse reactions manifest themselves in the form of symptoms of gastrointestinal disorders and disturbances in water and electrolyte balance.
In case of renal failure, an overdose provokes crystalluria (formation of sand in the urinary tract) and nephrotoxicity (toxic damage to the kidneys). Therapeutic assistance consists of taking activated carbon (1 tablet per kilogram of weight); in difficult cases, the patient is sent for hemodialysis. A specific antidote against the antibiotic has not been developed.
AMOXICILLIN (tablets)
some kind of potion from this medicine.
In general, in Yaroslavl we left with nothing. But in Uglich we still managed to purchase the medicine. For comparison, I will say that Amoxiclav cost about 400 rubles, that is, due to the acid included in its composition, it costs several times more. So, let's look at our antibiotic Amoxcillin, which we managed to purchase for only 64 rubles. His dosage is 500 mg. Well, in general we were recommended 500 + 125 mg or 625 mg. But there was only one there, and we no longer had time to think. We had to take what they gave us because we were already late for our ship. We were recommended to take antibiotics for 5 days, and it helped. The reason I had to take it was an infection that caused a large boil to form above my eyebrow and cause swelling in my eye. When we were still in Moscow, we managed to see a doctor, so he prescribed me to take this medicine. We did not take any bifidobacteria, and nothing happened. The antibiotic was tolerated quite easily and we did not notice any side effects, and the boil, meanwhile, began to decrease.
This medicine is produced “which is located in the city of Kirov by order of the Ecoland-Krylatskoye company.
The shelf life of this medicine is three years, as indicated on the box.
It says here that this drug should be taken only as prescribed by a doctor and that it is actually available with a prescription. Well, we had an appointment, but for some reason they didn’t give us a prescription.
This box contains 20 tablets of this medicine.
There are instructions in the box and they are as big as a sheet. Moreover, it is written on both sides in small print. So I advise you to familiarize yourself with it before taking this medicine. It is not necessary to read everything, and people far from medicine will understand little of it, but you must read the contraindications and side effects. Here's what the instructions look like:
And this is her rear view:
So the instructions are quite large in size, and such huge canvases always scare me, but since the doctor prescribed it, then I need to drink it. Moreover, all these boils cannot be picked, but this happened to us, so it was impossible to do without an antibiotic. And this is what the tablets look like. They are quite large and divisible, that is, you can take only half of such a tablet:
Here we can look at the blister with tablets from the back side. It says their name and dosage, and also that there are 20 of these tablets in the package.
In general, summing up the results of using this drug, I would like to say that it helped us a lot. But I do not advise you to neglect bifidobacteria. This is not a fact that nothing will happen. It is still better to take these same biphyobacteria when taking antibiotics. Here's what the tablet itself looks like:
Here we see that our tablets are divisible:
I will recommend this antibiotic to everyone, but only with a doctor’s prescription, and ask for a prescription. Better yet, don’t get sick, which is what I sincerely wish for everyone. I wish everyone that they would have to take antibiotics less often. I give this medicine an A because it helped us. Good drug.
Dosages according to instructions, methods of therapy with Amoxicillin
The specifics of treatment depend on the form of the drug. The antibacterial medication is taken regardless of meal time and must be washed down with water.
For Amoxicillin tablets, the following rules apply:
- For patients over 12 years of age, 0.5 g of the drug is prescribed three times a day. The final dosage depends on the complexity of the ongoing pathological process; in case of severe lesions, it increases to 0.75-1 g. The maximum permitted daily volume of the medication does not exceed 6 g.
- Large dosages are recommended for the treatment of typhoid fever (1.5 to 2 g per day), leptospirosis (0.5 to 0.75 g four times a day). After the disappearance of clinical manifestations of the disease, treatment continues for 48-72 hours. The total therapy time lasts 5-12 days.
Amoxicillin suspension is intended for children no older than 5 years. The solution is prepared before use: water at room temperature is added to the bottle, the mixture is vigorously shaken. The finished substance retains its properties for 2 weeks. Shake the medicine before each dose. The dosage is measured using a 5 ml measuring spoon containing 0.25 g of the drug.
For children under 2 years old, 0.02 g per kilogram of body weight is prescribed, from 2 to 5 years old - 0.125 g of the drug. Children under 10 years of age are prescribed 0.25 g of antibiotic, older ones - from 0.25 to 0.5 g of medication. In difficult cases, the dose may be increased to 1 g.
Amoxicillin preparations: how to make the right choice?
In modern conditions of the progressive development of antibiotic resistance, the choice of effective drugs and antibacterial treatment regimens is difficult. In this regard, it is extremely important to identify certain predictors of the effectiveness of antimicrobial action, which would increase the chances of an adequate response when prescribing an antimicrobial drug. The main goal of research in recent years has been to identify the relationship between the minimum inhibitory concentration (MIC) of antibiotics against the infectious agent and the pharmacokinetic and pharmacodynamic parameters of the drug. Today, it is obvious that there are certain pharmacokinetic parameters, the assessment of which allows one to reliably predict bacteriological effectiveness (pathogen eradication in more than 80% of cases). Thus, for beta-lactams and macrolides (except azithromycin), it is known that the concentration of the antibiotic at the site of infection should exceed its MIC against the pathogen for more than 40% of the time from the interval between drug administrations (T > MIC). For azithromycin and fluoroquinolones, it has been proven that the ratio of the area under the curve (AUC) to the MIC value should be greater than 25 (AUC/MIC), and for severe infections this value should exceed 125. The ratio of the maximum serum concentrations to MIC [1].
The reliability of the prognostic significance of the proposed parameters has been confirmed in numerous in vivo experiments and clinical observations [2].
This approach is applicable to all antibiotics, including new ones, since on its basis it is possible to optimize the treatment regimen at the early stages of preclinical and clinical studies, reduce the risk of developing adverse drug reactions, and prevent the formation of resistance. Thus, the choice of an antibiotic, maximizing the regimens and schemes for its use in the clinic includes a number of stages: assessment of the spectrum of action and antimicrobial activity; determination of the main parameters of pharmacokinetics (PK) and pharmacodynamics (PD), selection of the most effective antibiotic from a number of evaluated ones; Recommendation of optimal treatment regimens.
Amoxicillin remains one of the most widely used antibacterial drugs, which has significant pharmacokinetic advantages over a number of other penicillins. Thus, the bioavailability of amoxicillin reaches 70–80% (up to 95% in the form of Solutab), while for phenoxymethylpenicillin this most important pharmacokinetic indicator is 50%, and for ampicillin - only 40%. The degree of binding to plasma proteins for amoxicillin, on the contrary, is minimal and amounts to 17% (phenoxymethylpenicillin - 80%, ampicillin - 22%). High bioavailability and low degree of binding to plasma proteins provide a high concentration of amoxicillin in tissues when taken orally, which makes it possible to achieve eradication of the pathogen and at the same time avoid the development of adverse events. An important advantage of amoxicillin is the independence of absorption from the gastrointestinal tract from food intake, in contrast to phenoxymethylpenicillin and ampicillin, the bioavailability of which is significantly reduced when taken simultaneously with food. In pediatric practice, it seems important to have a soluble oral form. The favorable organoleptic properties of the soluble form of amoxicillin can improve patient compliance and tolerability of antibacterial therapy [3].
On the pharmaceutical market, amoxicillin is represented both by the drug in the original dosage form Flemoxin Solutab and by generic drugs. Considering the above data on the influence of pharmacokinetics on the final antibacterial effect of the drug, it is extremely interesting to compare the pharmacokinetic parameters of the drug in the original dosage form and generic analogues.
It is important to understand that the assessment of the pharmacokinetic properties of the antibiotics under study can be made both in clinical studies and in a number of situations, when conducting a dissolution test and comparability of dissolution kinetics. In accordance with current regulatory recommendations, dissolution kinetics are studied at various pH conditions (typically pH 1.2, 4.5, 6.8). To obtain a complete profile of dissolution kinetics, sampling intervals should be fairly frequent (at least every 15 minutes), and even more frequent sampling is recommended during periods of maximum change in dissolution kinetics.
If the active substance is highly soluble, the dosage form dissolves quickly at physiological pH values, and the excipients do not affect bioavailability, then it is assumed that there is no reason to study bioavailability in vivo.
For quantitative comparability of dissolution kinetics, an assessment of the value of the similarity factor is used, the value of which from 50 to 100 confirms the comparability of dissolution kinetics [4].
The purpose of our work was to conduct a test of comparative dissolution kinetics of various amoxicillin drugs as a way to assess the comparability of the pharmacokinetic profile of these drugs.
We conducted a study of the comparative dissolution kinetics of the drug Flemoxin Solutab - dispersible tablets, 500 mg, with three generic drugs of amoxicillin in the form of regular tablets in the same dosage. In addition, a quantitative determination of the amoxicillin content in the above preparations was performed.
The studies were carried out in the testing laboratory of NAKFF LLC using existing modern equipment. All studies were carried out according to ND 42–14184–06.
The study of comparative dissolution kinetics was carried out in accordance with the basic methodology and in accordance with the requirements of the State Pharmacopoeia XI, no. 2, on a paddle mixer at pH 1.2; 4.5; 6.8. The determination was carried out in accordance with the requirements of OFS 42–0003–04, using equipment from VARIAN, model “Varian Dissolution Test VK 7025”. The amount of dissolved amoxicillin was determined by spectrophotometry at an absorption maximum at a wavelength of 271 nm in a cuvette with a layer thickness of 10 mm, using a Varian Cary 100 Scan spectrophotometer.
Research results
Based on the results obtained, graphs of the dissolution kinetics of the drug Flemoxin Solutab and other generic drugs of amoxicillin were constructed (Fig. 1–3).
Quantitative assessment of the equivalence of the dissolution kinetics of drugs was carried out in accordance with the “Guide to the Expertise of Medicines, Volume I” of the Ministry of Health of the Russian Federation, Scientific Center for the Expertise of Medicinal Products, Moscow, 2013 [4].
As a result of the calculations, the results presented in table were obtained. 1.
An assessment of the values of the similarity factors given in the table allows us to conclude that the dissolution kinetics of all comparison drugs in three media is not equivalent to the dissolution kinetics of the drug Flemoxin Solutab dispersible tablets, 500 mg (Astellas Pharma Europe B.V., the Netherlands).
In addition, we carried out a quantitative determination of amoxicillin in the studied drugs (Table 2).
As follows from the presented data, in comparison drugs No. 1 and No. 2, the amount of the active component (amoxicillin) does not meet the requirements of ND 42-14184-06.
Discussion of the results obtained
The rational use of medicines is one of the most effective tools for optimizing the use of the healthcare budget, in the structure of which in some countries the costs of medicines have reached 30–40%. One of the main ways to reduce the cost of treatment is to replace original drugs (brands) with their generic analogs after the expiration of patent protection. Within the framework of subsidized medicine in all countries, generics are given preference over original drugs. In the European Union alone, generic drugs have been able to “save” (redistribute) 20 billion euros annually over the past 20 years [5].
The widespread use of generics in medical practice in our country is a benefit that allows us to provide medical care to a larger number of patients at lower material costs, since generic drugs are always cheaper (sometimes tens of times) compared to the original ones. The use of generic drugs in certain situations really helps to save budget money and cover a larger number of patients with modern pharmacotherapy. However, in this situation, it is absolutely necessary to know that the generic and the original drug are therapeutically equivalent - that is, they must be pharmaceutically equivalent and can be expected to have the same clinical effect and the same safety profile when used by patients.
As noted earlier, even a slight change in the properties of a generic drug can lead to a change in specific antibacterial activity, a decrease in tissue concentration and a weakening of the therapeutic effect. Evidence of this was given in a number of works [6–8].
In our work, using a well-known standardized test, we analyzed the comparability of dissolution parameters (and therefore pharmacokinetic parameters) of various amoxicillin preparations present on the Russian pharmaceutical market. We have found that the drug Flemoxin Solutab in the form of dispersible tablets has more stable dissolution kinetics with less dependence on the acidity of the medium compared to other studied generics of amoxicillin.
In addition, it has been proven that most of the generics studied have a lower content of active substance per tablet than in the Flemoxin Solutab dispersible tablet. It is obvious that even a slight reduction in the dose of the active substance entering the systemic circulation will inevitably affect the clinical effectiveness of the drug used. It is important to understand that reducing the dose of antibiotic entering the systemic circulation also contributes to the selection of resistance among microorganisms [1]. At the same time, one of the reference drugs (tablets of reference drug No. 1) showed the content of the active component to be higher than the upper limit required by the requirements of ND 42-14184-06, which may increase the risk of overdose.
Thus, as a result of our study, we can conclude that none of the studied drugs of amoxicillin can be considered fully equivalent to the drug Flemoxin Solutab, and therefore, it can be assumed that the use of the studied drugs may be accompanied by either less clinical efficacy, or a higher risk of developing adverse events compared to the drug Flemoxin Solutab.
Literature
- Sidorenko CB Problems of etiotropic therapy of community-acquired respiratory tract infections // Consilium medicum. 2002; vol. 4, no. 1, 4–10.
- Craig WA Antimicrobial resistance issues of the future // Diagn. Microbiol. Infect. Dis. 1996: 25, 213–217.
- Chan DS, Demers DM, Bass JW Antimicrobial liquid formulations: a blind taste comparison of three brands of penicillin VK and three brands of amoxicillin // Ann Pharmacother. 1996; 30 (2): 130–132.
- Guidelines for the examination of medicinal products. T. I. M.: Grif i K., 2013.
- Nightingale CH A survey of the quality of generic clarithromycin product from 13 countries // Clin. Drug Invest. 2000. V. 19. P. 293–305.
- Samsygina G. A. Efficacy of various fluconazole preparations in the treatment of mucocutaneous candidiasis in children in the first weeks of life // Children's Doctor. 2001, No. 3, p. 40–41.
- Nikulin A. A., Tsyuman Yu. P., Martinovich A. A., Eidelshtein M. V., Kozlov R. S. On the issue of interchangeability of intravenous forms of original and generic drugs: are comparative studies needed? // Wedge. microbiol. antimicrobial chemotherapy 2010; 12 (1): 31–40.
- Martinovich A. A., Eidelshtein M. V., Tsyuman Yu. P., Kozlov R. S. Azithromycin: comparison of the quality of injectable dosage forms of the original drug and its generic drugs // Klin. microbiol. antimicrobial chemotherapy 2011. T. 14, no. 4. pp. 252–258.
S. K. Zyryanov*, Doctor of Medical Sciences, Professor Yu. B. Belousov*, Doctor of Medical Sciences, Professor A. V. Kamaev**, 1 A. A. Lelishentsev**
* GBOU VPO RNIMU im. N. I. Pirogova Ministry of Health of the Russian Federation, Moscow ** National Agency of Clinical Pharmacology and Pharmacy, Moscow
1 Contact information