The use of amoxicillin for community-acquired pneumonia in children
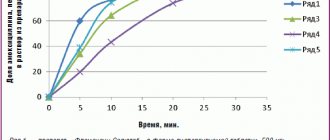
Community-acquired pneumonia (CAP) is one of the most pressing problems of modern medicine and consists of a number of epidemiological, clinical, pharmacological and, finally, social aspects. The paradox of pneumonia is that, on the one hand, impressive results have been achieved in understanding the pathogenesis of the infectious process and increasing the effectiveness of chemotherapy, and on the other hand, there is an increase in the number of patients with severe disease and mortality [3]. Over the course of a year, more than 17 million people are diagnosed with pneumonia in the Russian Federation. The risk group includes children under 5 years of age and elderly people over 65 years of age [4]. Pneumonia is the leading cause of death in children worldwide. Every year, pneumonia claims the lives of about 1 million children under the age of five (in 2015 - 920,136 children, 15% of all child deaths in the world) [1]. In Russia, the incidence of CAP is growing every year. Thus, in 2016, the incidence of community-acquired pneumonia in adults and children was 418.02 per 100 thousand population, which is 24% higher than in the previous 2015 (337.1, respectively) (Fig.).
As in previous years, in 2016, the maximum incidence of CAP was observed in children in the age group of 1–2 years (1456.7 per 100 thousand). The mortality rate from pneumonia in 2016 in the Russian Federation was 4.9 per 100 thousand population, and in some regions it reached 18.8–22.1 per 100 thousand population (in the Altai Republic, Tyumen, Sakhalin, Kirov, Amur regions. ) [19]. According to the Ministry of Health of the Russian Federation, respiratory diseases in children aged 0–17 years occupy third place in the structure of causes of death after external causes and developmental defects. In 2016, the mortality rate of children under 17 years of age from community-acquired pneumonia was 0.4 per 100 thousand population, while over the past 5 years, no significant changes in this indicator for children under 17 years of age have been registered [19].
Community-acquired pneumonia should be understood as an acute disease that arose outside of a hospital or was diagnosed in the first 48 hours from hospitalization, or developed in a patient who was not in nursing homes/long-term medical observation units for more than 14 days, manifested by symptoms of systemic inflammation (fever, shortness of breath, tachycardia, leukocytosis), impaired health (refusal to eat, drowsiness, inappropriate behavior), signs of infectious lesions of the lower respiratory tract (cough, sputum production, chest pain at the height of inspiration) and radiological signs of “fresh” focal infiltrative changes in lungs in the absence of an obvious diagnostic alternative [3].
Pneumonia is an infectious disease associated with the penetration of microorganisms into the respiratory system. The resulting inflammatory reaction in the lung parenchyma depends on the number and virulence of the microorganism, the state of the protective mechanisms of the respiratory tract and the body as a whole. The main route of penetration of the microorganism into the lung tissue is aerogenic. The microorganism, having overcome the protective barriers of the respiratory tract, can enter directly into the alveoli and multiply there intensively. Under the influence of microbial toxins, the permeability of capillaries is disrupted, and serous edema develops. Edema fluid, containing a large number of bacteria, quickly spreads through the alveolar pores to the entire lobe of the lung, often involving the pleura in the inflammatory process. The exudate quickly turns from serous to fibrinous, and the affected part of the lung becomes dense. Impaired bronchial patency, microcirculation disorders, inflammatory infiltration, interstitial edema of the pulmonary parenchyma and decreased airiness of the pulmonary parenchyma lead to impaired gas perfusion and hypoxemia; the latter is accompanied by respiratory acidosis, hypercapnia, compensatory shortness of breath and the appearance of clinical signs of respiratory failure [5].
In accordance with ICD-10 and the “Classification of Clinical Forms of Bronchopulmonary Diseases in Children,” the following forms of pneumonia are distinguished by etiology: bacterial, viral, fungal, parasitic, chlamydial, mycoplasma, mixed [6, 7]. The incidence of bacterial pneumonia in 2016 was 16.5 times higher than viral pneumonia (112.4 and 6.8 per 100 thousand population, respectively).
According to morphological forms, they distinguish: focal, focal-confluent, segmental, polysegmental, lobar and interstitial pneumonia. In the focal form, one or several foci of pneumonic infiltration of 1–2 cm in size occur.
- Focal-confluent (pseudolobar infiltrate) is a heterogeneous massive pneumonic infiltration, consisting of several foci. May be complicated by destructive processes and exudative pleurisy. Segmental - pneumonia, the boundaries of which repeat the anatomical boundaries of the 1st segment.
- Polysegmental - pneumonia, the boundaries of which repeat the anatomical boundaries of several segments. It often occurs with a decrease in the size of the affected area of the lung (atelectatic component).
- Lobar (lobar) pneumonia - the inflammatory process covers a lobe of the lung. A variant of the course of lobar pneumonia is lobar pneumonia.
- Interstitial - along with inhomogeneous infiltrates of the pulmonary parenchyma, there are pronounced, sometimes predominant changes in the interstitium of the lungs. A rare form of pneumonia that develops in patients with immunodeficiency conditions.
According to the flow, CAP is distinguished with an acute course (lasting up to 6 weeks) or protracted (lasting more than 6 weeks). The chronic course of pneumonia is currently not considered and is not included in the “Classification of clinical forms of bronchopulmonary diseases in children” [6, 7].
Based on severity, EP is classified into moderate and severe. The severity of CAP is determined by the severity of clinical manifestations and the presence of complications. Complications: pleural (pleurisy), pulmonary (cavitary formations, abscess), pulmonary-pleural (pneumothorax, pyopneumothorax), infectious-toxic shock [6, 7].
In children 3 months - 5 years old, CAP is most often caused by S. pneumoniae (according to some studies, their share is 70-88% of cases [8, 9]). Of the typical bacteria, H. influenzae type b also plays a role (up to 10% of cases, mainly in children under 2 years of age).
Diagnosis of CAP based on clinical symptoms is associated with significant difficulties. The diagnosis of pneumonia must be made at the patient’s bedside, but it is considered reliable only if infiltration of the lung tissue is detected on a chest x-ray and the presence of at least two of the following criteria [11]:
1) fever above 38 °C for three or more days; 2) cough with sputum; 3) physical symptoms of pneumonia (local weakening of breathing and fine moist rales); 4) leukocytosis > 15 × 109/L and/or the number of band neutrophils > 10%.
To decide on hospitalization and determine the required volume of medical care, it is necessary to assess the severity of CAP. In table Table 2 presents the key criteria for assessing the severity of CAP in children depending on age, proposed by the European Respiratory Society (ERS) [13].
Risk factors for death from pneumonia in children [10]:
- age under 5 years and male gender;
- unfavorable premorbid background of children;
- low socio-economic status of the family;
- late seeking medical help;
- late admission to hospital.
In the vast majority of cases (about 80%), children with CAP can be effectively treated at home. Indications for hospitalization are [11]:
- age up to 6 months of life;
- severe form of the disease;
- the presence of severe underlying diseases - congenital heart disease, chronic lung diseases accompanied by infection (bronchopulmonary dysplasia, cystic fibrosis, bronchiectasis, etc.), immunodeficiency, diabetes mellitus;
- carrying out immunosuppressive therapy;
- lack of conditions for treatment at home or guarantees of implementation of recommendations - socially disadvantaged family, poor social conditions (dormitory, orphanage, temporary accommodation center, etc.);
- lack of response to initial antibiotic therapy (ABT) within 48 hours (maintenance of high fever, increasing respiratory failure, appearance of agitation or depression of consciousness).
ABT has a decisive influence on the prognosis of pneumonia [5, 12]. The choice of ABT in each case of CAP is carried out individually, taking into account the natural activity of the drugs against the pathogen and its possible acquired resistance, the severity and course of the disease, and the presence of contraindications in the patient to the use of certain antibiotics. The principle of choosing ABT depending on the causative agent of CAP is presented in Table. 3.
In clinical practice, especially in outpatient settings, empirical ABT is more often performed, taking into account the most likely pathogen and its sensitivity in the region, the patient’s age, the presence of background diseases, toxicity and tolerability of antibacterial drugs (ABP) for a particular patient.
Evidence-based studies have shown that the use of oral amoxicillin for uncomplicated CAP in children aged 3 months to 5 years is not inferior in effectiveness to benzylpenicillin or ampicillin administered intravenously [15, 16]. In this regard, in all children with CAP who do not have indications for hospitalization, as well as in hospitalized children with moderate CAP, it is advisable to use oral ABT.
In children over 3 months of age, the main antibiotic for the treatment of CAP is amoxicillin (at a standard dose of 45-50 mg/kg per day), since this antibiotic has high, stable activity against the most common and dangerous pathogen - S. pneumoniae, as well as in most cases it is active against H. influenzae [13, 15, 17]. The level of amoxicillin in tissues (including the lungs) is significantly higher than equal doses of phenoxymethylpenicillin and ampicillin. It has a longer half-life, so it is prescribed 2-3 times a day. Food does not affect the bioavailability of amoxicillin, which is important when prescribing to children: unlike oral penicillin, amoxicillin can be given to a child both before and after meals.
When choosing a dosage form, it should be taken into account that the capsule has better bioavailability compared to amoxicillin in tablet form (93% and 70–80%, respectively), which helps to increase efficiency and reduce the risk of adverse events from the intestines.
Amosin® (amoxicillin) is a broad-spectrum antibiotic from the group of semisynthetic penicillins. It disrupts the synthesis of peptidoglycan in the bacterial cell wall during division and growth and causes bacterial lysis. 1–2 hours after administration it reaches maximum concentration in plasma, sputum, bronchial secretions, and pleural fluid. Therapeutic concentrations of amoxicillin are maintained in plasma for 8 hours after oral administration. Amosin® is available in the form of capsules of 250 mg, tablets of 250 and 500 mg and powder for oral suspension of 125, 250 and 500 mg. A new form of Amosin® tablets No. 20 (250 mg) and No. 20 (500 mg) has also appeared. The form of single-dose sachets facilitates the process of preparing the suspension and significantly expands the possibilities of using the drug in children: easy to use (no need to store the prepared suspension in the refrigerator), better It is digestible, allows you to choose the optimal dose depending on body weight and has a pleasant fruity taste. The sachet form is convenient for people with difficulty swallowing.
For children under 5 years of age, Amosin® is prescribed in the form of a suspension. The dose for children under 2 years of age is 20 mg/kg/day in 3 divided doses. For children aged 2 to 5 years, Amosin® is prescribed 125 mg 3 times a day; at the age of 5 to 10 years - 250 mg 3 times a day. Adults and children over 10 years of age weighing more than 40 kg are prescribed 500 mg 3 times a day. The maximum dose for severe manifestation of clinical symptoms is 0.75–1 g 3 times a day.
The sensitivity of S. pneumoniae to amoxicillin remains at a very high level - 99.6%, while the level of resistance to penicillin exceeded the critical value - 10%, to ciprofloxacin and ceftibuten - more than 6%, to cefixime - 5% [14].
For CAP caused by typical bacteria, the duration of therapy is usually 7–10 days, and for atypical bacteria – 10–14 days [13, 15, 17, 18]. ABT can be completed 3–4 days after stable normalization of body temperature [18].
20 children with community-acquired pneumonia aged 5 to 14 years were treated on an outpatient basis in the outpatient department of the State Budgetary Healthcare Institution of the NSO Children's Clinical Hospital No. 6 in Novosibirsk in 2016–2017, of which there were 12 boys (60%) and 8 girls (40%) . Children sought medical help 1–5 days after the onset of pneumonia; on average, the first visit to the doctor took place 2.3 days after the onset of clinical symptoms.
During an objective examination, all children were diagnosed with “community-acquired pneumonia”, of which 18 (90%) had a typical X-ray picture, consisting of focal damage to the lungs, in the remaining 2 children (10%) pneumonia turned out to be X-ray negative, the diagnosis was confirmed by computed tomography data lungs.
The complex of treatment measures included antibacterial therapy with Amosin®, mucolytic agents, physical therapy and therapeutic exercises. Amosin® was used as monotherapy in an age-related dose with a frequency of use 3 times a day according to domestic recommendations for 7–10 days. The average duration of taking Amosin® was 8.3 days. At the same time, stabilization of the clinical condition (effective therapy) was achieved in 90% of children receiving Amosin®. Their clinical symptoms have regressed; children with acute focal pneumonia are not subject to X-ray monitoring, since with positive clinical dynamics, complete resorption of the infiltrate usually occurs after 2–3 weeks. There was no discontinuation of Amosin® due to the development of adverse events.
Thus, the clinical effectiveness of drug therapy, including taking the drug Amosin®, was assessed as recovery in the majority of children diagnosed with community-acquired pneumonia. The above allows us to recommend Amosin® (amoxicillin) for use in outpatient pediatric practice as the drug of choice for acute focal pneumonia.
Literature
- WHO AND UNICEF Bulletins. Electronic resource: https://www.who.int/mediacentre/factsheets/fs331/ru/.
- Vovk E.I., Vertkin A.L. Community-acquired pneumonia at the beginning of the 21st century: the cost of living in a big city // Attending Doctor. 2008. No. 8. pp. 63–65.
- Mustafin T.I., Kudoyarov R.R. Current issues of community-acquired pneumonia // Medical Bulletin of Bashkortostan, 2014. No. 5. P. 39–41.
- World health statistics 2015. World Health Organization 2015. 164 p.
- Community-acquired pneumonia in children. Clinical recommendations, M., 2015. 64 p.
- Geppe N. A., Rozinova N. N., Volkov I. K., Mizernitsky Yu. L. Working classification of the main clinical forms of bronchopulmonary diseases in children. Russian Respiratory Society, 2009. 18 p.
- Geppe N. A., Rozinova N. N., Volkov I. K., Mizernitsky Yu. L. Modern classification of clinical forms of bronchopulmonary diseases in children. // Pediatrics. 2010; 89 (4): 6–15.
- Crawford SE, Daum RS Bacterial pneumonia, lung abscess and empyema/Pediatric respiratory medicine/Ed. Taussig LM, Landau LI Mosby, Inc., 2008: 501–553.
- Tatochenko V.K. Clinical recommendations. Pediatrics (Pneumonia in children) / Ed. A. A. Baranova. M.: GEOTAR-Media, 2005. 28 p.
- Mizernitsky Yu. L., Sorokina E. V., Ermakova I. N. et al. Organization of medical care for children with pneumonia in the Russian Federation // Ros. Vestn. perinatol and pediatrics. 2005; 3:4–8.
- Community-acquired pneumonia in children. Clinical recommendations. M.: Original layout, 2015. 64 p.
- Punpanich W., Groome M., Muhe L. et al. Systematic review on the etiology and antibiotic treatment of pneumonia in human immunodeficiency virus-infected children // Pediatr. Infect. Dis. J. 2011. Vol. 30. No. 10. R. 192–202.
- Esposito S., Patria MF, Tagliabue C. et al. CAP in children/European respiratory monograph 63: Community-acquired pneumonia/Ed. J. Chalmers, M. Pletz, S. Aliberti. 2014. R. 130–139.
- Kozlov R. S. Pneumococci: lessons from the past - a look into the future. Smolensk: MAKMAKH, 2010. 128 p.
- Harris M., Clark J., Coote N. et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011 // Thorax. 2011. Vol. 66, Suppl. 2–23.
- Das RR, Singh M. Treatment of severe community-acquired pneumonia with oral amoxicillin in under-fi ve children in developing countries: a systematic review // PLoS One. 2013. Vol. 25. No. 6. e66232.
- Bradley JS, Byington CL, Shah SS et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America // Clin. Infect. Dis. 2011. Vol. 53. No. 7. e25–76.
- Chuchalin A.G., Sinopalnikov A.I., Kozlov R.S., Tyurin I.E., Rachina S.A. Community-acquired pneumonia in adults: practical recommendations for diagnosis, treatment and prevention: a manual for doctors. M., 2010. 106 p.
- On the state of sanitary and epidemiological well-being of the population in the Russian Federation in 2016: State report. M.: Federal Service for Supervision of Consumer Rights Protection and Human Welfare, 2022. 220 p.
V. V. Provorova1, Candidate of Medical Sciences E. I. Krasnova, Doctor of Medical Sciences, Professor L. M. Panasenko, Doctor of Medical Sciences, Professor V. G. Kuznetsova, Doctor of Medical Sciences, Professor N. G. Paturina, Candidate of Medical Sciences sciences
Federal State Budgetary Educational Institution of Higher Education NSMU Ministry of Health of the Russian Federation, Novosibirsk
1 Contact information
Rational antibacterial therapy of pneumonia
How quickly should antibiotic therapy be started for pneumonia? What is the duration of antibacterial therapy and what does it depend on? Does it make sense to use a combination of two antibiotics and under what circumstances is such therapy used?
The problem of antibacterial therapy (AT) for pneumonia is still relevant, since frequent strategic and tactical errors in the treatment of this disease have a significant impact on its outcome. The presence of a large arsenal of antibacterial drugs (ADs), on the one hand, expands the possibilities of AT for various infections, and on the other hand, requires the doctor not only to be aware of numerous ARs (spectrum of action, pharmacokinetics, side effects, etc.), but also to have the ability to navigate issues of microbiology, clinical pharmacology and other related disciplines.
Prescribing and carrying out AT for pneumonia requires the doctor to take a whole range of measures, and each of his decisions determines the effectiveness of the prescribed treatment. When prescribing AT, the doctor should be guided by the following key parameters:
- selection of initial AP for empirical AT;
- dose and route of administration of AP;
- assessment of the effectiveness of the initial AP;
- adequate replacement of ineffective AP;
- AT duration;
- possibility of stepped AT;
- justification for the need for combined AT;
- assessment of toxicity and tolerability of AP.
Selecting the initial AP
AT should begin as early as possible, from the moment pneumonia is diagnosed. According to some data, if the administration of the first dose of AP is delayed by more than 8 hours from the moment of hospitalization, there is a significant increase in mortality among elderly and senile patients. The need for antibiotics to be prescribed as early as possible (before receiving the results of a microbiological study) is due to:
- rapid decompensation of concomitant pathology;
- worsening prognosis;
- lack of sputum or difficulties in obtaining it for examination in some situations;
- frequent negative results of sputum examination;
- difficulties in interpreting the data obtained (colonization of respiratory mucous membranes, sputum contamination);
- the inability to isolate certain pathogens from sputum (mycoplasma, legionella).
The main guidelines when choosing an initial AP for the treatment of pneumonia are:
- clinical and epidemiological situation;
- antimicrobial spectrum of action of the selected drug;
- sputum Gram stain results;
- AP pharmacokinetics;
- trend and likelihood of antibiotic resistance;
- severity of pneumonia;
- AP safety in a specific situation;
- possibility of step therapy;
- AP cost.
The “situational approach” when choosing the initial antibiotic for the treatment of pneumonia is justified by the “attachment” of some pathogens of pneumonia to certain clinical and epidemiological situations. In addition, the prescription of AT is carried out immediately after diagnosis in the absence of data from microbiological examination of sputum, and often without the prospect of etiological verification of the etiological variant of pneumonia.
Perhaps the greatest number of errors occurs at the very beginning of AT, at the stage of empirical therapy, when choosing an AP. Most often, errors are associated with underestimation or incorrect assessment of clinical and epidemiological situations, X-ray and laboratory data, which suggest an approximate etiological variant of pneumonia. It is necessary to remember the different etiologies of pneumonia in young and elderly people, in previously healthy patients and patients with various background pathologies, in those with pneumonia at home or in the hospital, in patients in the surgical or intensive care unit, etc. Lack of clear criteria for choosing the initial AP leads to the fact that the doctor is guided by completely different subjective criteria, for example, he gives preference to the most familiar, well-known and frequently prescribed AP, or, conversely, prescribes a new, more effective, in his opinion, AP, or opts for a cheaper and available AP, etc. For example, cephalosporins with antipseudomonas activity (ceftazidime, cefpirome) or antipseudomonas penicillins (mezlocillin, carbenicillin) are erroneously prescribed for community-acquired pneumonia of non-severe course in young patients not burdened with any concomitant pathology. In this case, the most likely etiological agents, along with pneumococcus, may be the so-called atypical pathogens (legionella, mycoplasma, chlamydia). It is not justified to prescribe antibiotics such as vancomycin or meropenem, which are considered “reserve”, to a patient with mild community-acquired pneumonia. This approach not only contradicts the principles of choosing the initial AM, but is also economically irrational. In addition, a medical error in this case is fraught with the formation of microbial resistance to these APs. In the above situation of community-acquired pneumonia in a young patient who is not burdened with concomitant pathology, it is more justified to prescribe aminopenicillins (amoxicillin, ampicillin) or macrolides (erythromycin, azithromycin, clarithromycin, spiramycin), which are also active against probable atypical pathogens (Legionella, Chlamydia, Mycoplasma). This allows for the most complete coverage of all etiologically significant pathogens (third generation cephalosporins, vancomycin and meropenem are not active against atypical pathogens). In table Table 2 provides possible reasons for the ineffectiveness of AT and methods for their correction.
Among fluoroquinolone APs for community-acquired pneumonia, the prescription of new fluoroquinolones (levofloxacin, moxifloxacin), which have high activity against S. pneumoniae, H. influenzae and atypical pathogens, is justified. At the same time, the prescription of gram-negative fluoroquinolones (ciprofloxacin, ofloxacin) is irrational, since these APs have low antipneumococcal activity.
On the other hand, when choosing an AP for the treatment of hospital-acquired pneumonia, one should focus on the high probability of the etiological role of gram-negative microorganisms, including P. aeruginosa (late pneumonia in intensive care units, long-term treatment with glucocorticoids, etc.). In such situations, it is more justified to prescribe third-generation cephalosporins with antipseudomonas activity (ceftazidime), or antipseudomonas penicillins (piperacillin), or fluoroquinolones (ciprofloxacin)
The pharmacokinetic properties of drugs can serve as another guideline when choosing an initial AP. The main pharmacokinetic properties that must be taken into account when choosing an antigen:
- concentration in lung tissue and alveolar macrophages;
- bioavailability of the drug when taken orally;
- half-life duration - dosage regimen;
- the presence of a post-antibiotic effect;
- no interaction with other medications;
- routes of elimination from the body.
Macrolides, tetracyclines and fluoroquinolones penetrate well into tissues. When penetrating into the cell, the drug should not cause damage, which is most characteristic of tetracyclines. The penetration of macrolides into the cell is so pronounced that their extracellular concentrations may be insufficient to suppress pneumococci in pneumococcal bacteremia. Taking this into account, in severe pneumonia with a high probability of bacteremia, monotherapy with macrolides is unjustified.
The pharmacokinetic properties of some APs (ciprofloxacin, third and fourth generation cephalosporins, etc.) allow their use no more than 2 times a day. The optimal pharmacokinetic parameters of new (respiratory) fluoroquinolones (levofloxacin, moxifloxacin), their high, almost complete bioavailability when taken orally, make it possible to prescribe them once a day, both parenterally and orally.
Antibiotic resistance. When choosing an AP for empirical AT of pneumonia, one should take into account the tendency of a number of microorganisms to antibiotic resistance (AR) and the regional “microbial ecological situation”, that is, the predominant spectrum of microorganisms and their sensitivity to antibiotics in different regions, hospitals, departments, etc.
One of the main problems of clinical importance is the resistance of S. pneumoniae to penicillin. The risk of penicillin resistance in S. pneumoniae increases in the presence of the following factors: the age of patients is less than 7 years and older than 60 years, the presence of severe somatic diseases, frequent and long-term treatment with antibiotics, and living in nursing homes. Cross-resistance to macrolides is possible. At the same time, the resistance of S. pneumoniae to penicillin and macrolides does not correlate with resistance to respiratory fluoroquinolones, which makes the choice of respiratory fluoroquinolones (levofloxacin, moxifloxacin) rational and justified in such situations. Resistance of S. pneumoniae to levofloxacin remains low and does not exceed 0.8%. According to the recommendation of the American Thoracic Society, it is levofloxacin and moxifloxacin that are approved for use in community-acquired pneumonia caused by resistant S. pneumoniae.
Another problem that arises in connection with antibiotic resistance is the production of b-lactamase by H. influenzae, which is usually observed in patients with COPD, who are often treated with AP in connection with exacerbations of the disease. Taking into account this factor, in the development of pneumonia against the background of COPD, the prescription of protected penicillins (amoxicillin/clavulanate, ampicillin/sulbactam) is justified. Since the mechanisms of penicillin resistance in S. pneumoniae and H. influenzae are different (membrane changes and b-lactamase production, respectively), protected penicillins are active against b-lactamase-producing H. influenzae and are ineffective against penicillin-resistant S. pneumoniae. At the same time, “protected” penicillins can remain active against penicillin-resistant staphylococci (staphylococci produce b-lactamase). Therefore, in situations where the likelihood of staphylococcal community-acquired pneumonia is high (after influenza, chronic alcohol intoxication), the prescription of inhibitor-protected penicillins is justified.
Of great clinical importance is the identification of resistance in patients with nosocomial pneumonia (methicillin-resistant S. aureus), which determines the tactics of AT and serves as a rationale for prescribing vancomycin. At the same time, the choice of the latter as an empirical AT for even severe pneumonia, as mentioned above, should be considered erroneous, and its prescription should be justified by the isolation of resistant S. aureus.
It is irrational to prescribe co-trimoxazole or tetracycline as an initial AP for community-acquired pneumonia due to the high level of resistance of the main pathogens of pneumonia to these APs.
Gram staining of sputum is an important guideline for choosing the initial antibiotic, taking into account the Gram affiliation of microorganisms. It is advisable to perform bacterioscopy and culture of sputum containing a sufficient number of neutrophils. A negative result with Gram stain of sputum does not always indicate the absence of microorganisms in the sputum, but may be due to an insufficient number of them (less than 104). If about 10 microorganisms are detected in one field of view, this means that their number is at least 105 and is approaching the diagnostic titer.
AP cost. When initially choosing an antibiotic, its cost should be considered taking into account the adequacy of the antibiotic in a given situation, as well as the additional costs of treatment in case of ineffectiveness, development of complications, undesirable effects, etc. It should be borne in mind that not only the cost of the antibiotic itself is important, but and the total costs of treatment, which, if a cheaper but ineffective AP is prescribed in this situation, may be higher.
An increase in treatment costs is usually associated with an incorrect initial choice of AP, combined AT without proper indications, inadequate duration of AT, and underestimation of the risk of undesirable toxic effects of AP.
AP dose and route of administration
Often, inadequate doses of AP are used to treat pneumonia, and both insufficient and excessive doses of the drug can be prescribed. If the dose of AP is insufficient and the concentration of the drug necessary to eradicate the corresponding pathogen is not created in the blood, then this is not only one of the reasons for the ineffectiveness of AP, but also creates real preconditions for the formation of resistance of the microorganism. Errors in choosing the optimal dose can be associated both with the prescription of an insufficient single dose and with an incorrect dosage regimen (insufficient frequency of administration).
The incorrect choice of intervals between AP administrations is usually due not so much to the difficulties of parenteral administration of drugs in an outpatient setting or the negative attitude of patients, but rather to the lack of awareness of doctors about the pharmacodynamic and pharmacokinetic features of APs, which should determine their dosage regimen. Thus, a number of APs have a so-called post-antibiotic effect, that is, the ability to suppress the growth of microorganisms even when the level of APs in the blood decreases below the MIC. Aminoglycosides, fluoroquinolones, and tetracyclines have such a postantibiotic effect against gram-negative microorganisms. The severity of the bactericidal effect of these groups of APs depends mainly on the peak concentration in the blood, therefore, when prescribing these drugs, it is important that the patient receives a sufficient single dose, and the intervals between administrations may be longer. On the other hand, b-lactam APs, with the exception of carbapenems, are practically devoid of post-antibiotic effect. Their bactericidal effect does not increase with increasing levels of drugs in the blood. Therefore, when choosing b-lactam APs (penicillins, cephalosporins), for their optimal action, long-term maintenance of MIC in the blood is much more important, that is, more frequent (without omissions) administration of the drug. Taking into account the above, two- or three-time administration of penicillins or cephalosporins of the first and second generation, even in an adequate single dose, should be considered erroneous. On the other hand, when prescribing aminoglycosides, a sufficient single dose is required, which can be administered even once. Prescribing AP in doses exceeding the optimal ones can cause the development of superinfection due to the activation of the patient’s own microflora. Superinfection usually occurs when prescribing APs that reduce the level of non-spore anaerobic flora in the intestine (penicillins, lincomycin, tetracyclines). In this case, usually, after a short-term effect against the background of AT, there is an increase in temperature and a deterioration in the patient’s condition, which is mistakenly interpreted as the ineffectiveness of AT and entails an unjustified replacement of AP, which in turn also does not have the expected effect.
It should be remembered that the use of large doses of AP increases the risk of toxic effects. This applies primarily to potentially toxic drugs such as aminoglycosides, for which strict daily doses have been established. Exceeding such “threshold” doses is unacceptable, especially in patients with a high risk factor for complications (elderly and senile age, impaired renal function, taking other potentially nephrotoxic drugs, etc.)
The administration of large doses of AP may, however, be justified when there is a high risk of resistant strains or when a pathogen with moderate resistance to the corresponding AP is isolated. Thus, it is possible to prescribe large doses of amoxicillin (up to 3 g/day) for pneumonia caused by penicillin-resistant pneumococcus, since penicillins and cephalosporins can retain their effectiveness.
The route of administration of AP is determined by many factors, including the severity of pneumonia, the patient’s condition, the pharmacokinetic characteristics of AP, etc. If in a number of situations with lower respiratory tract infections, the parenteral route of administration has no alternative (impaired consciousness, brainstem disorders with impaired swallowing, intestinal pathology etc.), then in other cases parenteral AT requires certain indications and should be justified and not arbitrary. The doctor’s desire to “facilitate and simplify” treatment (both for the patient and for the nursing staff), prescribing APs orally without taking into account the specific clinical situation and pharmacokinetics of APs, may become one of the reasons for the insufficient effect or even ineffectiveness of treatment of pneumonia. AP should not be prescribed orally for severe pneumonia, especially for drugs with low bioavailability (ampicillin, cefuroxime axetil), which do not allow achieving the optimal concentration of AP in the blood. At the same time, in patients with mild community-acquired pneumonia in the absence of complications and severe background pathology, oral AT is acceptable. In such situations, the parenteral route of administration of AP is not only unjustified, but also more expensive. Meanwhile, oral forms of some APs are not widely used in the treatment of community-acquired pneumonia. For example, the frequency of parenteral and oral use of cephalosporin antibiotics in Russia is 94.2 and 5.8%, respectively.
Evaluation of the effectiveness of the initial AP
The critical period for assessing the effectiveness of AP is considered to be 48-72 hours from the moment of its prescription. Typically, the criterion for the effectiveness of AP is a decrease or normalization of the patient’s body temperature and a decrease in signs of intoxication. In cases where fever from the very beginning of the disease is not pronounced or is completely absent, one has to focus on other signs of intoxication (headache, anorexia, cerebral symptoms, etc.), as well as on the absence of progression of the disease during the period of treatment.
Unfortunately, we often have to deal with the fact that the patient continues to receive the prescribed AP for a week or more, despite the absence of an obvious clinical effect. Continuing AT, despite its ineffectiveness, is fraught with many negative consequences. At the same time, the prescription of another, more adequate AP is delayed, which contributes to the progression of pulmonary inflammation (which is especially important in severe pneumonia and in patients with concomitant pathologies), the development of complications, and prolongation of treatment. In addition, the risk of side (toxic) effects of AT and the development of antibiotic resistance increases. One should not ignore the fact that the ineffectiveness of the therapy entails a loss of confidence in the doctor of the patient, as well as his relatives. We also cannot discount the economic costs associated with inadequate prescription of APs (wasteful consumption of ineffective APs, excessively long hospital stay for the patient, additional costs for treating the toxic effects of APs, etc.).
There are also errors associated not only with assessing the effectiveness of AT, but also with replacing an ineffective AM with another, that is, with changing the AM. In the absence of microbiological research data, the principle of choosing an AP remains the same, that is, one has to focus on the clinical situation, taking into account the ineffectiveness of the initial AP and other additional signs. The lack of effect from the initial AP, to a certain extent, should serve as an additional guideline for choosing a second AP. For example, the lack of effect from b-lactam APs (penicillins, cephalosporins) in a patient with community-acquired pneumonia suggests the presence of legionellosis or mycoplasma pneumonia (taking into account, of course, other signs). In turn, this can be considered as a justification for prescribing APs from the group of macrolides (erythromycin, azithromycin, spiramycin, clarithromycin, etc.) or new generation fluoroquinolones (levofloxacin, moxifloxacin).
Combined AT
Today, when doctors have a wide arsenal of APs at their disposal, the indications for combined AT are significantly narrowed and the priority in AT for pneumonia remains with monotherapy. The main indications for prescribing combined AT are severe pneumonia, a high probability of mixed flora, the presence of pneumonia against the background of severe immunodeficiency (malignant tumors, treatment with cytostatics and immunosuppressants, etc.). Unfortunately, we have to deal with situations in which patients with mild pneumonia, in the absence of complications or severe background pathology, are prescribed two APs. The prescription of two APs is usually justified by the arguments that each of the APs has a different spectrum of action and there is a greater chance of quickly achieving a therapeutic effect in the conditions of empirical AT.
The advisability of combining macrolides with cephalosporins in the treatment of severe pneumonia is due to the likelihood of Legionella pneumonia and the difficulties of its etiological verification. It has been shown that mortality in community-acquired pneumonia, especially among elderly patients, is lower when prescribing combination therapy with second- and third-generation cephalosporins in combination with macrolides compared with monotherapy with third-generation cephalosporins. However, mortality with monotherapy with modern respiratory fluoroquinolones (levofloxacin) is also lower than with monotherapy with third-generation cephalosporins.
The negative aspects of unjustified combined AT are the selection of multiple resistant strains of microorganisms and the occurrence of superinfection, an increased risk of developing toxic effects due to the fact that the adverse effects of the drugs are cumulative, as well as an increase in the cost of treatment. The combined administration of APs that are excreted by the kidney is especially undesirable, since such therapy creates a real risk of nephrotoxic effects. Moreover, with irrational combinations, a decrease in the effectiveness of therapy due to AP antagonism is possible. An example of irrational combinations are such fixed combinations of APs as oletethrin and tetraolean (drugs are practically not used at present), in which the macrolide oleandomycin is contained in an insufficient dose, and tetracycline cannot be used in most cases as an initial AP for the treatment of pneumonia. The insufficient dose of oxacillin and ampicillin contained in the combined drug Ampiox does not allow us to recommend this drug for community-acquired pneumonia, including cases of suspected staphylococcal etiology of pneumonia.
Duration of AT pneumonia
The main goal of AT is primarily to eradicate the pathogen or inhibit its further growth, that is, to suppress microbial aggression. The duration of AT can be determined by many factors, including the etiological variant of pneumonia, the presence of complications, etc.
In cases of uncomplicated community-acquired pneumococcal pneumonia, the duration of AT is 7-10 days. Legionella and mycoplasma pneumonia require long-term AT - up to 3 weeks, since these etiological agents have intracellular localization. In case of complicated pneumonia, often caused by staphylococcus (pulmonary destruction, empyema, abscesses), the duration of AT should also not be less than 3 weeks.
The actual inflammatory reaction of the lung tissue, which is manifested by various clinical and radiological signs (auscultation pattern, persistent radiological infiltration, acceleration of ESR), regresses more slowly and does not require continued AT. In this regard, the tactics according to which a patient with persistent radiological signs of pulmonary infiltration, crepitating rales (crepitacio redux), an increase in ESR with normalization of body temperature and the disappearance (or decrease) of signs of intoxication continue to receive AT should be considered erroneous. An even more serious mistake is replacing one AP with another in such situations, which the doctor classifies as the ineffectiveness of the initially prescribed AT. In some patients, after the disappearance of signs of intoxication and even regression of inflammatory changes in the lungs, weakness, sweating, and low-grade fever may persist for a long time. The latter is often mistakenly regarded by the doctor as a manifestation of an incompletely controlled bronchopulmonary infection, which is also “confirmed” by radiological data in the form of changes in the pulmonary pattern or “residual effects of pneumonia” and usually entails the continuation of AT or additional prescription of AP, despite the absence of changes in the side of the blood. Meanwhile, such a clinical situation is often caused by autonomic dysfunction after a pulmonary infection (post-infectious asthenia) and does not require AT, although, of course, in each specific case a thorough examination of the patient and decoding of all existing symptoms are necessary. Unreasonably prolonged AT of pneumonia increases the risk of developing superinfection, microbial resistance, side and toxic effects of AP, and also increases treatment costs. Situations with delayed radiological resolution of pneumonia require special consideration.
Step therapy
The so-called step-by-step therapy is unreasonably rarely used, which involves parenteral administration of an AP as the first stage, and then, after achieving a clinical effect, a transition to the oral route of administration of the same AP. This opportunity exists when choosing APs that have both parenteral and oral dosage forms. Studies have shown that stepwise therapy does not worsen the results of treatment of pneumonia and the prognosis of the disease. The obvious advantages of stepwise AT are providing greater comfort of treatment, reducing hospitalization time and the ability to continue treatment on an outpatient basis, as well as reducing treatment costs
Among the factors influencing the effectiveness of stepwise AT are low bioavailability of the drug, impaired intestinal absorption, and the risk of patient non-compliance with the dosage regimen. However, these disadvantages can be avoided in most cases.
The main requirements for the transition from parenteral to oral administration of AP are the following:
- the presence of antibiotics in oral and parenteral forms;
- effect of parenterally administered antibiotic;
- stable condition of the patient;
- the ability to take drugs orally;
- absence of intestinal pathology;
- high bioavailability of oral antibiotic.
Many modern APs available in the doctor’s arsenal meet these requirements, including macrolides (erythromycin, azithromycin), respiratory fluoroquinolones (levofloxacin, moxifloxacin), which, along with other properties (spectrum of antimicrobial activity, pharmacokinetics, safety), makes it possible to consider their use rational for community-acquired pneumonia.
Prevention and control of side effects and toxic effects
When prescribing and conducting AT for pneumonia, increased attention should be paid to the safety of AP, and therefore numerous factors (age, concomitant pathology, taking other medications, etc.) that determine the tactics of AT should be taken into account. Inadequate assessment of the characteristics of a given patient entails the development of side toxic effects. Most often, errors can occur in the following situations:
- age of patients (children, old people);
- pregnancy;
- severe concomitant pathology with functional disorders of various organs and systems;
- drug therapy for concomitant diseases;
- allergic reactions to various APs.
Pregnant women should not be prescribed fluoroquinolones, clindamycin, metronidazole. In addition, aminoglycosides, vancomycin, and imipenem should be used with caution.
The presence of concomitant pathology in some cases creates difficulties in carrying out AT, which can lead to errors in the choice of AP, its dosage, routes of administration, duration of AT, etc. The errors may be based either on the failure to identify concomitant pathology or its underestimation in relation to toxic effects of the AP, or, finally, insufficient knowledge of the pharmacokinetic characteristics of the selected AP. If a patient has renal failure, preference should be given to choosing an antiretroviral agent with predominantly extrarenal elimination (cefoperazone) or with a dual elimination route (ciprofloxacin). It is erroneous to prescribe potentially nephrotoxic APs (aminoglycosides, carbapenems) without dose adjustment in patients with concomitant renal failure. It is also dangerous in such situations to use a combination of APs that have nephrotoxic properties (aminoglycosides and cephalosporins, with the exception of cefoperazone).
Particular attention should be paid to the presence of concomitant, often multiple pathologies with functional disorders of organs and systems in elderly and senile patients. An age-related decrease in glomerular filtration rates, along with a high incidence of nephroangiosclerosis in the elderly, should be considered as one of the factors influencing the choice of AP, which, unfortunately, is not always taken into account in clinical practice.
In the presence of reliably established hypersensitivity to penicillin, the prescription of other b-lactam APs (cephalosporins, carbapenems) should be considered an error. As an alternative to AP, fluoroquinolones and macrolides can be prescribed. However, it should be borne in mind that reactions of another origin (vascular, vegetative, etc.) are often mistaken for an allergy to antibiotics, and therefore patients’ indications of such “intolerance” should be critically assessed and the existing situation should be analyzed more carefully. However, intradermal tests for AP are dangerous, since there is the same risk of severe anaphylactic reactions.
Thus, managing a patient with pneumonia requires the doctor to make key decisions taking into account currently available standards of treatment and appropriate adjustment of the prescribed AT, depending on the clinical situation. The algorithm for managing a patient with community-acquired pneumonia is presented in the figure.
Coronavirus pneumonia. My history. Matvey, 45 years old.
Matvey, 45 years old, who was treated in April 2022 with a diagnosis of bilateral polysegmental pneumonia, covid+, talks about how the disease developed, what he had to endure and what helped him recover.
I am at home now. Discharged on April 14. Spent 2 weeks in the hospital. I feel great now! There was no temperature in the hospital in recent days. He was discharged with positive dynamics based on the CT results, as the doctors say. Stopped taking medications while in the hospital.
When I arrived home, I called a doctor from the clinic. The doctor listened to the lungs and was satisfied. Now I'm in self-isolation.
WHERE IT ALL BEGAN
That day, upon returning home from work, I felt severe chills - apparently my temperature was rising. I knew how the coronavirus manifests itself. I immediately told my family about everything, isolated myself - conditions allowed, I lived in the country. And until the day I was hospitalized, I had no contact with anyone, this saved my entire family.
On the first night there was a terrible chill, the temperature was a little 37.2 ° C, but the sensation was there - it was just pounding. Nothing helped. The next day it was 37.4°C in the morning, this temperature remained the whole day and the next three days. I already understood that this was not an ordinary ARVI and was looking for an opportunity to do a test for coronavirus, but it didn’t work.
All this time I was at home, periodically lowering my temperature (not too high), but I felt worse and worse. When I realized that the saddle test at home would not work, I called an ambulance. The doctor examined me and prescribed me an antibiotic and an antipyretic.
Around day 5-6, I developed a cough and pain in the chest when I inhaled—I couldn’t take a deep breath. The temperature rose to 38.5°C. Then I decided to do a CT scan myself, at a nearby clinic. A CT scan showed signs of bilateral lung damage, characteristic of viral pneumonia, and it became clear that she needed to be hospitalized.
Arriving in Moscow, I called an ambulance, which took me to City Clinical Hospital No. 52. I had the CT result in hand, so I spent a little time in the emergency department. They took tests, conducted additional examinations, and filled out medical documentation. A ready-made CT scan on hand greatly reduces your stay in the emergency room. Now, as far as I know, this can be done on an outpatient basis, in a clinic. Many clinics accept patients with signs of acute respiratory viral infection, perform a CT scan, and if they see changes in the lungs, they are immediately hospitalized.
WHERE COULD I GET INFECTED?
I haven't been abroad this year. It should be noted that all my friends who came from abroad wore masks so as not to infect others, realizing that they could be a source of infection without knowing it.
When information began to appear that people were getting sick with this infection, I realized that I had to take personal measures - I limited contacts, asked my friends if anyone had gotten sick. Yes, I continued to go to the gym until it closed, but I was sure that the risk here was small, since I tried to keep my distance and did not intersect with other visitors.
I visited the store 2-3 times. I didn’t wear a mask, I admit. I was sure that sick people should wear a mask so as not to infect others.
By the way: When I rode in a car with a driver, I was wearing a mask and he was wearing a mask - so far he has not gotten sick. Important: if you suspect that you may be a carrier of the infection, it is better to isolate yourself from others. When I felt something was wrong, I immediately isolated myself from my family - no one got sick either then or now. It's good when you have the opportunity to distance yourself - it can save the lives of your loved ones. It is very important to follow the rules of personal hygiene - wear a mask in public places, wash your hands thoroughly. It works, believe me.
IN THE HOSPITAL
I admit, I thought about being treated in a paid hospital. But when I found myself in hospital No. 52 and from the first minute I was surrounded by the care of wonderful doctors and nurses, I realized that I was unlikely to stay here, but I wanted to be treated here.
I was placed in a multi-bed ward; there were only two patients in it, me and the emergency doctor. The doctor came, examined my neighbor and me, and made prescriptions. The temperature at that moment was about 39°C, they put me on a drip and prescribed meds. The state was like Vysotsky’s in the song - he injected himself and forgot.
The head of the department (we didn’t know each other then) said that based on the CT results they suspected I had a coronavirus infection, and asked me not to go out into the corridor and not to contact other patients. I understood that this was important - we were as isolated as possible, as far as the hospital conditions allowed.
The next day, as it turned out, taking into account all my data, I was informed that the doctors wanted to prescribe me a treatment method that had previously been used in rheumatology patients, but based on the experience of treating coronavirus infection in other countries, it can be effective for severe lesions lungs to stop the process. I had to give consent, and I gave it. I understood perfectly well that in war all means are good if it comes to saving lives. A medical consultation was held, which decided to prescribe this type of treatment to me, after which I was put on a drip for an hour and a half.
HOW I RECOVERED
The next day, T 39.9°C turned into 37.4°C, and my health improved significantly. My feeling is that a fierce war was going on in my body, and suddenly it ended. Until that day, it was extremely difficult for me to breathe, I felt relief only when I used an oxygen mask, and then suddenly the saturation (oxygen saturation in the blood) rose to 95%.
I breathed oxygen the entire next day (by the way, every bed has access to oxygen). The internal tension gradually disappeared, I directly felt relief coming. Saturation reached 97%. On the doctor’s recommendation, I breathed oxygen while lying on my stomach for 30 minutes twice a day—the saturation reached 98–99%. After a couple of days, the temperature dropped to 36.8oC, I began to feel much better; I had a second CT scan, and 2 days later a third, and it was from this that it became clear that there was a positive trend. The tests were normal and I was taken off all medications.
IT'S ALL BEHIND NOW
And so on April 14, I was discharged to continue treatment at home. I continue the self-isolation regime, but now everything is happening much calmer, less alarming - no one from my circle has gotten sick, and I don’t think they will get sick. Everyone observed the self-isolation regime, lived in the country, and did not communicate with anyone.
I am very grateful to the 52nd hospital, very much. True professionals, wonderful doctors. They work selflessly without days off. They treat patients like family. I will definitely meet with them all as soon as possible, I wish the quarantine would end sooner.
The press service then asked me a question: will I then recognize my saviors without masks?
Remember, there was a movie like this during our youth - “You for me, I for you”? Kuravlev was a bathhouse attendant, known in narrow circles, and treated high-ranking persons. It was possible to get to him only through great connections. And somehow by chance, his former client meets him on the train: “Ivan Sergeevich, is that you?” - I don’t recognize something, turn your back... Syroezhkin?? - He is!
So, when we meet in peacetime, you just have to look into their beautiful eyes, I will recognize them with a 99.9% probability, you can be sure.
* The author's text is published with the consent of the patient.