Valentin Vlasov, Vera Morozova, Igor Babkin, Nina Tikunova “Science at First Hand” No. 4(70), 2016
About the authors
| Valentin Viktorovich Vlasov — Academician of the Russian Academy of Sciences, Doctor of Chemical Sciences, Professor, Director of the Institute of Chemical Biology and Fundamental Medicine of the Siberian Branch of the Russian Academy of Sciences (Novosibirsk). Laureate of the State Prize of the Russian Federation (1999). Author and co-author of more than 300 scientific papers and 20 patents. |
| Vera Vitalievna Morozova — Candidate of Biological Sciences, senior researcher at the Laboratory of Molecular Microbiology at the Institute of Chemical Biology and Fundamental Medicine SB RAS (Novosibirsk). Author of more than 30 scientific papers and 6 patents. |
| Igor Viktorovich Babkin — Candidate of Biological Sciences, leading researcher at the Laboratory of Molecular Microbiology at the Institute of Chemical Biology and Fundamental Medicine SB RAS (Novosibirsk). Author and co-author of 58 scientific papers and 2 patents. |
| Nina Viktorovna Tikunova — Doctor of Biological Sciences, Head of the Laboratory of Molecular Microbiology at the Institute of Chemical Biology and Fundamental Medicine SB RAS (Novosibirsk). Author and co-author of 120 scientific papers and 21 patents. |
In the middle of the last century, biological science took a revolutionary step forward, establishing the molecular basis for the functioning of living systems. Bacteriophages, discovered at the beginning of the last century, played a huge role in successful research that led to the determination of the chemical nature of hereditary molecules, deciphering the genetic code and creating technologies for gene manipulation. Today, these bacterial viruses have mastered many useful “professions” for humans: they are used not only as safe antibacterial drugs, but also as disinfectants and even as a basis for the creation of electronic nanodevices.
When in the 1930s a group of scientists took up the problems of the functioning of living systems, then in the search for the simplest models they paid attention to bacteriophages
- bacterial viruses. After all, among biological objects there is nothing simpler than bacteriophages, moreover, they can be easily and quickly grown and analyzed, and viral genetic programs are small.
A phage is a minimally sized natural structure containing a tightly packed genetic program (DNA or RNA), in which there is nothing superfluous. This program is enclosed in a protein shell equipped with a minimal set of devices for its delivery inside the bacterial cell. Bacteriophages cannot reproduce on their own, and in this sense they cannot be considered full-fledged living objects. Their genes begin to work only in bacteria, using the biosynthetic systems available in the bacterial cell and the reserves of molecules necessary for synthesis. However, the genetic programs of these viruses are not fundamentally different from the programs of more complex organisms, so experiments with bacteriophages made it possible to establish the fundamental principles of the structure and operation of the genome.
Subsequently, this knowledge and the methods developed during the research became the foundation for the development of biological and medical science, as well as a wide range of biotechnological applications.
Bacteriophages are our friends when it comes to bacteria pathogenic to humans. However, there are other bacteria that are friendly to us, which are used in modern biotechnological production, as well as in traditional food industry production, such as cheese making, etc. In these cases, phages can cause great harm, since in large populations of microorganisms that are in the stage intensive growth, favorable conditions are created for the reproduction of phages, which leads to the lysis of production bacterial cultures. In cheese production, the problem is not so serious, since in this case they usually use starters consisting of many cultures, some of which will withstand the phage attack and continue the process of lactic acid fermentation. Serious problems arise if the entire process is based on the use of one specific bacterial strain, as, for example, in the production of antibiotics or therapeutic proteins.
Pathogen Fighters
The first attempts to use bacteriophages to treat infectious diseases were made almost immediately after their discovery, but the lack of knowledge and imperfect biotechnologies of that time did not allow complete success to be achieved. Nevertheless, further clinical practice has shown the fundamental possibility of successfully using bacteriophages for infectious diseases of the gastrointestinal tract, genitourinary system, for acute purulent-septic conditions of patients, for the treatment of surgical infections, etc.
Compared to antibiotics, bacteriophages have a number of advantages: they do not cause side effects, and are also strictly specific for certain types of bacteria, so their use does not disrupt the normal human microbiome. However, such high selectivity also creates problems: in order to successfully treat a patient, you need to know exactly the infectious agent and select the bacteriophage individually.
Phages can also be used prophylactically. Thus, Moscow Research Institute of Epidemiology and Microbiology named after. G. N. Gabrichevsky developed a preventive product “FUDFAG” based on a cocktail of bacteriophages, which reduces the risk of contracting acute intestinal infections. Clinical studies have shown that weekly use of the drug allows you to get rid of hemolyzing Escherichia coli and other pathogenic and opportunistic bacteria that cause intestinal dysbiosis.
Bacteriophages are used to treat infectious diseases not only of humans, but also of domestic and farm animals: mastitis in cows, colibacillosis and escherichiosis in calves and pigs, salmonellosis in chickens... It is especially convenient to use phage preparations in the case of aquaculture - for the treatment of industrially farmed fish and shrimp, since They last a long time in water. Bacteriophages also help protect plants, although the use of phage technologies in this case is difficult due to the influence of natural factors, such as sunlight and rain, which are destructive to viruses.
Phages can play a big role in maintaining the microbiological safety of food products, since the use of antibiotics and chemical agents in the food industry does not solve this problem, while simultaneously reducing the level of environmental cleanliness of products. The seriousness of the problem itself is evidenced by statistical data: for example, in the USA and Russia, up to 40 thousand cases of salmonellosis are registered annually, of which 1% die. The spread of this infection is largely associated with the raising, processing and consumption of various types of poultry, and attempts to use bacteriophages to combat it have shown promising results.
Thus, the American company Intralytix
produces phage preparations to combat listeriosis, salmonellosis and E. coli bacterial contamination. They are approved for use as additives that prevent the growth of bacteria on food - they are sprayed on meat and poultry products, as well as on vegetables and fruits. Experiments have shown that a cocktail of bacteriophages can be successfully used in the transportation and sale of live pond fish to reduce bacterial contamination not only of the water, but also of the fish itself.
An obvious use of bacteriophages is disinfection.
, that is, the destruction of bacteria in places where they should not be: in hospitals, food production, etc. For this purpose, the British company
Fixed-Phage
has developed a method for fixing phage preparations on surfaces, ensuring the preservation of the biological activity of phages for up to three years .
Bacteriophages - “drosophila” of molecular biology
The experiment of American researchers A. Hershey and M. Chase using bacteriophages labeled with sulfur and phosphorus isotopes proved the role of DNA as the main carrier of genetic information.
In 1946, at the 11th symposium in the famous American laboratory in Cold Spring Harbor, the theory “ one gene - one enzyme." Bacteriologist A. Hershey and “former” physicist and molecular biologist M. Delbrück reported on the exchange of genetic characteristics between various phages while simultaneously infecting E. coli cells. This discovery, made at a time when the physical carrier of the gene was not yet known, indicated that the phenomenon of “recombination”—the mixing of genetic characteristics—is characteristic not only of higher organisms, but also of viruses. The discovery of this phenomenon subsequently made it possible to study in detail the molecular mechanisms of replication. Later, experiments with bacteriophages made it possible to establish the principles of the structure and operation of genetic programs.In 1952, A. Hershey and M. Chase experimentally proved that the hereditary information of bacteriophage T2 is encoded not in proteins, as many scientists believed, but in DNA molecules (Hershey & Chase, 1952). The researchers followed the reproduction process in two groups of bacteriophages, one of which carried radioactively labeled proteins, and the other carried DNA molecules. After infection of bacteria with such phages, it turned out that only viral DNA was transferred into the infected cell, which served as evidence of its role in the storage and transmission of hereditary information.
As objects for their research, M. Delbrück and his collaborators used mutant bacteriophages of the so-called T-series, which infect Escherichia coliIn the same year, American geneticists D. Lederberg and N. Zindler, in an experiment involving two strains of Salmonella and bacteriophage P22, established that the bacteriophage is capable of incorporating DNA fragments of the host bacterium during the reproduction process and transmitting them to other bacteria during infection (Zinder & Lederberg , 1952). This phenomenon of gene transfer from a donor bacterium to a recipient has been called "transduction". The results of the experiment became another confirmation of the role of DNA in the transmission of hereditary information.
In 1969, A. Hershey, M. Delbrück and their colleague S. Luria became Nobel laureates “for their discoveries concerning the mechanism of replication and the genetic structure of viruses.”
In 1972, R. Bird and colleagues, when studying the process of replication (copying cellular information) of E. coli DNA, used bacteriophages as probes capable of integrating into the genome of a bacterial cell, and discovered that the replication process occurs in two directions along the chromosome (Stent, 1974 ).
Advantages of bacteriophages
| Bacteriophages – antibacterial agents and natural antiseptics | Safe and non-toxic, no side effects, used in newborns, pregnant and lactating women |
| The action of bacteriophages does not affect the beneficial microflora of the body, unlike antibiotics | Bacteriophages are compatible with all medications. The use of bacteriophages does not limit the use of other drugs and does not affect their effectiveness |
| Affects only pathogenic bacteria that are sensitive to them and cause infectious disease, destroying them from the inside | Bacteriophages are eliminated from the body naturally |
Seven days of creation
Modern methods of synthetic biology make it possible not only to introduce various modifications into phage genomes, but also to create completely artificial active phages. Technologically, this is not difficult, you just need to synthesize the phage genome and introduce it into the bacterial cell, and there it will start all the processes necessary for the synthesis of proteins and the assembly of new phage particles. In modern laboratories this work will take only a few days.
Genetic modifications are used to change the specificity of phages and increase the effectiveness of their therapeutic effects. To do this, the most aggressive phages are equipped with recognition structures that bind them to the target bacteria. Also, genes encoding proteins that are toxic to bacteria and disrupt metabolism are additionally inserted into viral genomes—such phages are more lethal to bacteria.
Bacteria have several mechanisms of defense against antibiotics and bacteriophages, one of which is the destruction of viral genomes by restriction enzymes
, acting on specific nucleotide sequences. To increase the therapeutic activity of phages, due to the degeneracy of the genetic code, it is possible to “reformat” the sequences of their genes in such a way as to minimize the number of nucleotide sequences “sensitive” to enzymes, while simultaneously preserving their coding properties.
A universal way to protect bacteria from all external influences - so-called biofilms
, films of DNA, polysaccharides and proteins that bacteria create together and where neither antibiotics nor therapeutic proteins penetrate. Such biofilms are a headache for doctors, as they contribute to the destruction of tooth enamel, form on the surface of implants, catheters, artificial joints, as well as in the respiratory tract, on the surface of the skin, etc. To combat biofilms, special bacteriophages containing the gene , encoding a special lytic enzyme that destroys bacterial polymers.
Enzymes “from bacteriophage”
A large number of enzymes, now widely used in molecular biology and genetic engineering, were discovered as a result of research on bacteriophages.
The traditional cloning scheme (incorporation of foreign DNA) using a plasmid (an extrachromosomal genetic element inherent in many strains of bacteria) as a “vector” begins with cutting the plasmid DNA and cutting out the desired section of chromosomal DNA using a restriction enzyme. The DNA fragment being cloned is then inserted into a plasmid, which is introduced into the bacterium, making it capable of producing the foreign protein encoded in the inserted fragment.
One such example is restriction enzymes, a group of bacterial nucleases that digest DNA. Back in the early 1950s. It was found that bacteriophages isolated from the cells of one strain of bacteria often reproduce poorly in a closely related strain. The discovery of this phenomenon meant that bacteria had a system for suppressing the reproduction of viruses (Luria & Human, 1952). As a result, an enzymatic restriction-modification system was discovered, with the help of which bacteria destroyed foreign DNA that had entered the cell. The isolation of restriction enzymes (restriction endonucleases) gave molecular biologists an invaluable tool that allowed them to manipulate DNA: insert one sequence into another or cut out the necessary fragments of the chain, which ultimately led to the development of technology for creating recombinant DNA.
Another enzyme widely used in molecular biology is the DNA ligase of bacteriophage T4, which “crosslinks” the “sticky” and “blunt” ends of double-stranded DNA and RNA molecules. And recently, genetically modified versions of this enzyme with greater activity have appeared.
Most of the RNA ligases used in laboratory practice, which “cross-link” single-stranded RNA and DNA molecules, also originate from bacteriophages. In nature, they primarily serve to repair broken RNA molecules. Researchers most often use bacteriophage T4 RNA ligase, which can be used to “sew” single-stranded polynucleotides onto RNA molecules to label them. This technique is used to analyze the structure of RNA, search for binding sites of RNA with proteins, oligonucleotide synthesis, etc. Recently, thermostable RNA ligases isolated from bacteriophages rm378 and TS2126 have appeared among the routinely used enzymes (Nordberg Karlsson, et al., 2010; Hjorleifsdottir , 2014).
Some of another group of extremely important enzymes, polymerases, were also obtained from bacteriophages. For example, the very “precise” DNA polymerase of bacteriophage T7, which has found application in various fields of molecular biology, such as site-directed mutagenesis, but is mainly used to determine the primary structure of DNA.
Chemically modified phage T7 DNA polymerase was proposed as an ideal tool for DNA sequencing as early as 1987 (Tabor & Richardson, 1987). Modification of this polymerase has increased its efficiency several times: the rate of DNA polymerization reaches more than 300 nucleotides per second, so it can be used to amplify large DNA fragments. This enzyme became the precursor of sequenase, a genetically engineered enzyme optimized for DNA sequencing in the Sanger reaction. Sequenase is highly efficient and has the ability to incorporate nucleotide analogues into the DNA sequence, which are used to improve sequencing results.
The main RNA polymerases (DNA-dependent RNA polymerases) used in molecular biology - enzymes that catalyze the process of transcription (reading RNA copies from a DNA template) - also originate from bacteriophages. These include SP6, T7, and T3 RNA polymerases, named after the corresponding bacteriophages SP6, T7, and T3. All of these enzymes are used for the in vitro synthesis of antisense RNA transcripts, labeled RNA probes, etc.
The first completely sequenced DNA genome was the genome of phage φ174, over 5 thousand nucleotides in length (Sanger et al., 1977). This decoding was carried out by the group of English biochemist F. Sanger, the creator of the famous DNA sequencing method of the same name.
Polynucleotide kinases catalyze the transfer of a phosphate group from an ATP molecule to the 5′ end of a nucleic acid molecule, the exchange of 5′-phosphate groups, or the phosphorylation of the 3′ ends of mononucleotides. In laboratory practice, the most widely used polynucleotide kinase is bacteriophage T4. It is commonly used in experiments to label DNA with a radioactive isotope of phosphorus. Polynucleotide kinase is also used to find restriction sites, DNA and RNA fingerprinting, and synthesize substrates for DNA or RNA ligases.
In molecular biological experiments, bacteriophage enzymes such as phage T4 polynucleotide kinase, usually used for labeling DNA with a radioactive phosphorus isotope, DNA and RNA fingerprinting, etc., as well as enzymes that cleave DNA, which are used to obtain single-stranded DNA templates, are also widely used in molecular biological experiments. for sequencing and analysis of nucleotide polymorphism.
Using synthetic biology methods, it was possible to develop bacteriophages armed with the most sophisticated weapons that bacteria use against the phages themselves. We are talking about bacterial CRISPR-Cas systems, which are a complex of a nuclease enzyme that cleaves DNA and an RNA sequence that directs the action of this enzyme on a specific fragment of the viral genome. A piece of phage DNA, which the bacterium stores as a “memory” in a special gene, serves as a “pointer”. When a similar fragment is found inside a bacterium, this protein-nucleotide complex destroys it.
Having understood the mechanism of operation of CRISPR-Cas systems, the researchers tried to equip the phages themselves with similar “weapons”, for which purpose they introduced into their genome a complex of genes encoding a nuclease and addressing RNA sequences complementary to specific regions of the bacterial genome. The “target” may be genes responsible for multidrug resistance. The experiments were a complete success - such phages attacked the bacteria they were “tuned” to with great efficiency.
What does a bacteriophage consist of?
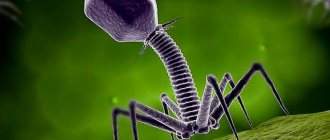
A typical phage consists of a “head” with a tightly packed genetic program consisting of nucleic acids (DNA or RNA), and a “tail” with which it “injects” its genes into the bacterial cell. The infected bacterium begins, using its own intracellular systems and resources, to synthesize proteins and nucleic acids necessary for the assembly of new viral particles. Mature phages go out in search of new prey, and the “parent” bacterial cell dies.
Thanks to recent research, it has become clear that bacteriophages play an important role in maintaining the global “microbial balance” in the biosphere: every two days they destroy half of the world’s bacterial population and thereby prevent these rapidly reproducing organisms from covering the earth’s surface with a thick layer.
Bacteriophages appear wherever bacteria live: on land and in the oceans, in soil and water, in plants and animals. Even the human gastrointestinal tract contains about 1012 bacteriophages - an order of magnitude more than the stars in our Galaxy! And although the size of phage particles does not exceed 0.0001 mm, the biomass of phages on the planet reaches a fantastic figure - 1 billion tons. Therefore, these invisible but omnipresent creatures are sometimes called the “dark matter” of the biosphere.
Phage antibiotics
Phages do not need to be used directly for therapeutic purposes. Over millions of years of evolution, bacteriophages have developed an arsenal of specific proteins - tools for recognizing target microorganisms and manipulating the biopolymers of the victim, on the basis of which antibacterial drugs can be created. The most promising proteins of this type are endolysin enzymes, which phages use to destroy the cell wall when leaving the bacterium. These substances themselves are powerful antibacterial agents that are non-toxic to humans. The effectiveness and direction of their action can be increased by changing their addressing structures - proteins that specifically bind to certain bacteria.
Most bacteria are divided according to the structure of their cell wall into gram-positive, whose membrane is covered with a very thick layer of peptidoglycan, and gram-negative, in which a layer of peptidoglycan is located between two membranes. The use of natural endolysins is especially effective in the case of gram-positive bacteria (staphylococci, streptococci, etc.), since their peptidoglycan layer is located on the outside. Gram-negative bacteria (Pseudomonas aeruginosa, Salmonella, Escherichia coli, etc.) are a less accessible target, since the enzyme must penetrate the outer bacterial membrane to reach the inner peptidoglycan layer.
To overcome this problem, so-called artilisins were created - modified versions of natural endolysins containing polycationic or amphipathic peptides that destabilize the outer membrane and ensure the delivery of endolysin directly to the peptidoglycan layer. Artilisins have high bactericidal activity and have already shown their effectiveness in the treatment of otitis media in dogs (Briers et al., 2014).
An example of a modified endolysin that selectively acts on certain bacteria is the drug P128 from the Canadian company GangaGen Inc.
. It is a biologically active fragment of endolysin combined with lysostaphin, a targeting protein molecule that binds to the surface of staphylococcal cells. The resulting chimeric protein has high activity against various strains of staphylococcus, including those with multidrug resistance.
Safe and effective
Phage therapy was born almost immediately after the discovery of bacteriophages themselves, but extensive testing of these antibacterial agents began to be carried out in the USSR only in the late 1930s. As a result, the effectiveness of bacteriophage preparations was proven as a prophylactic agent in the fight against epidemics of dysentery and cholera, and their use in the treatment of wounds and purulent-inflammatory processes showed their potential as an alternative to antibiotics.
However, the results of research at that time were often contradictory: sometimes phages immediately suppressed the development of infectious processes, but sometimes they turned out to be useless. Experts immediately understood the reason: the treatment was successful only when phages were used that could infect exactly the bacterial strain that caused the disease. Therefore, when an epidemic occurred, it was necessary to isolate an infectious agent, test the available phage preparations on it, and launch into production the most effective bacteriophage as a preparation.
Unfortunately, the results of such studies conducted in the USSR were not properly documented and described in the scientific literature, and they were carried out according to schemes that did not correspond to currently accepted clinical trial protocols. Nevertheless, the main results of this work were indisputable: phages proved their safety and high efficiency in real conditions and have since been used in our country in clinical practice along with conventional drugs.
Meet fecotransplantation
Clostridium difficile - sometimes rapidly multiply in the intestines.
, causing severe diarrhea that cannot be treated with medication. The problem is so serious that until recently, thousands of patients died from this disease in the United States every year. They learned to treat this diarrhea quite recently, and in a very simple way - by introducing fecal microflora taken from a healthy donor into the patient’s intestines. Recovery often occurs almost instantly, literally the next day. Obviously, with such a “transplant” of feces, the patient receives a full set of “correct” microorganisms destroyed by antibiotics, plus bacteriophages that regulate the number of pathogenic strains. Initially, the spread of the method in the United States was hampered by the FDA, which tried to apply regulatory principles adopted for conventional drugs in relation to it. However, protests from therapists and patients, along with the obvious safety of the procedure, played a role, and it was allowed to be carried out using the usual precautions - choosing healthy donors and performing the procedure by specialists in medical institutions. This treatment method has become widespread in the United States over the past few years, with good results. Probably, only the prejudice of doctors can explain the strange fact that fecotransplantation treatment is not practiced in all European countries today, and in Russia it can only be obtained at the Center for New Medical Technologies in the Novosibirsk Akademgorodok
With the advent of antibiotics, interest in phages in the West was lost, but after the emergence of antibiotic-resistant strains of bacteria in different countries, they began to develop phage preparations and conduct tests, which, in fact, repeated the research already carried out in the USSR. The results of these studies again confirmed the safety of bacteriophage preparations, which, in particular, was noted by the US Food and Drug Administration (FDA).
Pseudomonas aeruginosa , which is insensitive to antibiotics, with bacteriophages.
, and as part of the
Phagoburn
, seven medical centers in France, Belgium and Switzerland are conducting clinical trials of a cocktail of phages to prevent infections in burns.
Intralytix
,
Enbiotix
,
AmpliPhi
also report testing their own original phage cocktails for the treatment of a wide range of diseases . However, none of these large-scale clinical trials have yet been completed.
"Counters" of bacteria
Bacteriophages serve not only as a versatile therapeutic and “disinfectant” agent, but also as a convenient and accurate analytical tool for a microbiologist. For example, due to their high specificity, they are natural analytical reagents for identifying bacteria of a certain type and strain.
In the simplest version of such a study, various diagnostic bacteriophages are added dropwise to a Petri dish with a nutrient medium seeded with a bacterial culture. If the bacterium turns out to be sensitive to the phage, then in this place of the bacterial “lawn” a “plaque” is formed - a transparent area with killed and lysed bacterial cells.
By analyzing the reproduction of phages in the presence of target bacteria, it is possible to quantify the number of the latter. Since the number of phage particles in a solution will increase in proportion to the number of bacterial cells contained in it, to estimate the number of bacteria it is enough to determine the titer of the bacteriophage.
The specificity and sensitivity of such an analytical reaction are quite high, and the procedures themselves are simple to perform and do not require complex equipment. It is important that diagnostic systems based on bacteriophages signal the presence of a living pathogen, while other methods, such as PCR and immunoanalytical methods, only indicate the presence of biopolymers belonging to this bacterium. This type of diagnostic methods is especially convenient for use in environmental studies, as well as in the food industry and agriculture.
reference species are used to identify and quantify different strains of microorganisms
phages.
Very fast, almost real-time analytical systems can be created based on genetically modified bacteriophages, which, when they enter a bacterial cell, trigger the synthesis of reporter fluorescent (or luminescent) proteins, such as luciferase
. When the necessary substrates are added to such a medium, a luminescent signal will appear in it, the value of which corresponds to the content of bacteria in the sample. Such “light-labeled” phages were developed to detect dangerous pathogens such as plague, anthrax, tuberculosis, and plant infections.
It is likely that with the help of modified phages it will be possible to solve a long-standing problem of global importance - to develop cheap and fast methods for detecting tuberculosis pathogens at an early stage of the disease. This task is very difficult, since the mycobacteria that cause tuberculosis are characterized by extremely slow growth when cultivated in laboratory conditions. Therefore, diagnosing the disease using traditional methods can be delayed for up to several weeks.
Phage technology makes this task easier. Its essence is that bacteriophage D29, which is capable of infecting a wide range of mycobacteria, is added to the blood samples being analyzed. The bacteriophages are then separated and the sample is mixed with a fast-growing, non-pathogenic culture of mycobacteria that is also sensitive to this bacteriophage. If the blood initially contained mycobacteria that were infected with phages, then the production of bacteriophage will also be observed in the new culture. In this way, single mycobacterial cells can be detected, and the diagnostic process itself is reduced from 2–3 weeks to 2–5 days (Swift & Rees, 2016).
Phage therapy: through thorns
The discovery of bacteria-killing viruses gave rise to a new way to control bacterial populations. The most obvious application is phage therapy, the use of bacteriophages to treat human bacterial infections. Its advantage is the extreme specificity of phages, infecting only “selected” pathogens.
Bacteriophages were first used for medical purposes in 1915, when one of their discoverers, F. d'Herelle, used such a drug to treat dysentery in children. However, the subsequent history of the development of phage therapy was not easy. The fact is that d'Herelle's proposals were far ahead of their time, and for many years he had to fight for recognition of his discovery. Including famous French colleagues who did not recognize d'Herelle's point of view on the nature of these bactericidal agents, considering them enzymes. The truth triumphed only in the 1940s, but long before that, tired of the struggle, d'Herelle left to work in the USA.
In 1934, he came to Georgia, to Tbilisi, where by that time unique opportunities had developed for the development of phage therapy. Since 1918, there was a laboratory (later an institute) of microbiology, the head of which, G. Eliava, was sent to the famous Pasteur Institute to master methods and purchase equipment. It was there that he met d'Herelle and his amazing discovery.
Thus, Eliava’s dream was born to create a world center for bacteriophage research in Tbilisi. This idea interested I.V. Stalin, and in 1930 the building of the future Institute of Bacteriophages, Microbiology and Virology, which now bears the name of its founder, was built and equipped. However, further events developed according to a scenario characteristic of the USSR in those years: in 1937, Eliava and his wife were arrested and shot as an “enemy of the people,” and d’Herelle returned to Paris. However, the institute itself did not die and continued to function successfully.
Since the early 1940s. Phage therapy began to be used in Europe and the USA. Millions of patients have received such drugs, but the results of treatment have been contradictory and irreproducible. The advertisement promised miracles, but they did not happen - the very idea of phage therapy was compromised. The reason was that at that time, not only drug manufacturers, but also scientists themselves did not have the necessary knowledge about the properties of phages and their mechanism of action, and besides, there were no reliable technologies for working with viruses.
Failures followed failures, so it is not surprising that pharmacists and doctors breathed a sigh of relief with the advent of antibiotics. These relatively cheap, broad-spectrum antibacterial, well-storable chemicals seemed to radically solve the problem of treating infectious diseases. Bacteriophages were forgotten in the West for many years. The French company founded by d'Herelle for the production of commercial phage preparations switched to other projects (on its basis the famous cosmetics company L'Oreal grew).
Phage research continued only in the USSR, Poland and Czechoslovakia. The largest producer of phage preparations was the Georgian Institute created by Eliava: by the 1980s. About 1,200 people worked there, and the drugs were sent out for testing in clinics throughout the USSR. The production of bacteriophages was also organized in Ufa and Gorky.
By the way, in the cessation of work with bacteriophages abroad, in addition to the success of antibiotics, the political aspect also played a large role. After all, phage therapy was developed in the USSR, and it was politically “wrong” for Western scientists to work on topics related to the name of Stalin. Moreover, these were the times of Lysenkoism, when Western science perceived with skepticism everything that was done by Soviet biologists.
Phage display
Nowadays, bacteriophages are also widely used as simple systems for producing proteins with desired properties. We are talking about one developed in the 1980s. extremely effective molecular selection technique - phage display
. This term was proposed by the American J. Smith, who proved that, based on E. coli bacteriophages, it is possible to create a viable modified virus carrying a foreign protein on its surface. To do this, the corresponding gene is introduced into the phage genome, which is fused with the gene encoding one of the surface viral proteins. Such modified bacteriophages can be isolated from a mixture with wild-type phages due to the ability of the “foreign” protein to bind to specific antibodies (Smith, 1985).
Two important conclusions emerged from Smith's experiments: first, using recombinant DNA technology, it is possible to create hugely diverse populations of 106–1014 phage particles, each of which carries different protein variants on its surface. Such populations are called combinatorial phage libraries
. Secondly, by isolating a specific phage from a population (for example, one that has the ability to bind to a specific protein or organic molecule), this phage can be propagated in bacterial cells and an unlimited number of descendants with specified properties can be obtained.
Schematic diagram of the biopenning procedure—selection of highly specific recombinant antibodies to a specific target antigen from a combinatorial phage display library based on filamentous bacteriophages. From: (Tikunova, Morozova, 2009)
With the help of phage display, proteins are now produced that can selectively bind to therapeutic targets, for example, those exposed on the surface of the M13 phage, which are capable of recognizing and interacting with tumor cells. The role of these proteins in the phage particle is to “package” the nucleic acid, so they are well suited for creating gene therapy drugs, only in this case they form a particle with a therapeutic nucleic acid.
Today, there are two main areas of application of phage display. Peptide-based technology is used to study receptors and map antibody binding sites, create immunogens and nanovaccines, and map substrate binding sites of enzyme proteins. Technology based on proteins and protein domains - for selecting antibodies with specified properties, studying protein-ligand interactions, screening expressed fragments of complementary DNA and targeted modifications of proteins.
Using phage display, it is possible to introduce recognition groups into all types of surface viral proteins, as well as into the main protein that forms the body of the bacteriophage. By introducing peptides with specified properties into surface proteins, it is possible to obtain a whole range of valuable biotechnological products. For example, if this peptide imitates the protein of a dangerous virus or bacteria, recognized by the immune system, then such a modified bacteriophage is a vaccine that can be produced simply, quickly and safely.
If the terminal surface protein of the bacteriophage is “addressed” to cancer cells, and reporter groups (for example, fluorescent or magnetic) are attached to another surface protein, then a tool for detecting tumors will be obtained. And if a cytotoxic drug is also added to the particle (and modern bioorganic chemistry makes this easy to do), you will get a medicine that targets cancer cells.
One of the important applications of the phage protein display method is the creation of phage libraries of recombinant antibodies, where antigen-binding fragments of immunoglobulins are located on the surface of fd or M13 phage particles. Libraries of human antibodies are of particular interest, since such antibodies can be used in therapy without limitation. In recent years, about a dozen therapeutic antibodies constructed using this method have been sold on the US pharmaceutical market alone.
Phage cocktails
A new round of interest in phage therapy has occurred in recent years. The fact is that antibiotics have also not become a panacea for the treatment of bacterial infections: these days, the development of new drugs has not kept pace with the increase in the number of bacteria with acquired resistance to existing antibiotics. Already today, in hospitals in England, about 40% of staphylococcal infections are caused by such strains, and in the United States, about 90 thousand patients die annually from hospital infections caused by drug-resistant bacteria. When recalculated for the world's population, this number is 3-5 million deaths per year!
WHO warns that the world will soon enter a “post-antibiotic” era, when there will be no treatment for common bacterial infections. And against this background, phage therapy looks like a very promising direction, the development of which can lead to the creation of effective personalized methods for treating diseases. For this, there is both the necessary knowledge about phages and the mechanisms of their interaction with bacterial cells, as well as technologies for working with viral agents.
For phage therapy today, only virulent lysis phages are used, mainly “tailed” phages of the order Caudovirales, as well as filamentous phages of the families Leviviridae (with a single-stranded RNA genome) and Inoviridae (with a single-stranded circular DNA genome).
As discussed above, the activity spectra of phages are usually very narrow and limited to one or a few closely related bacterial species. On the one hand, such narrow specificity is good for therapy, since it allows you to eliminate a specific microorganism without disturbing the entire bacterial community of the human body. On the other hand, if emergency treatment is necessary (when there is no time to identify a specific bacterium causing the development of a pathogenic process in a wound or on a burned surface), it is necessary to have a drug that affects several types of bacteria, possible causative agents of infection. To solve this problem, phage cocktails are usually used - preparations containing several phages that differ in specificity.
This approach was also used by d'Herelle. D'Herelle's cocktail, which he brought from Paris back in 1930, is still one of the main phage preparations: it forms the basis of the Georgian pyophage and the Russian intestifage. In Tbilisi, based on phage cocktails, drugs were developed for the treatment of gastrointestinal diseases and purulent wounds for mass use in the event of epidemics or military operations. The results of army trials and a wide experiment on the prevention of childhood gastrointestinal disorders conducted in Tbilisi showed the good effectiveness of such drugs.
Phage cocktails are produced in a standardized manner and target bacterial communities commonly found in specific diseases. Of course, more effective cocktails are obtained when their components are matched to the bacterial community of a particular patient. To obtain such a cocktail, it is necessary to test the patient’s bacteria for sensitivity to phages from the collection in order to select the most effective phage strains. If the required phages are not in the collection, bacteria-specific phages are searched for in natural substrates.
In general, the search for bacteriophages is quite simple: a bacterial culture is exposed to samples from various sources: water bodies, soil, sewage, etc. If the bacteria die, they are separated from the solution by centrifugation, and the remaining solution is tested for activity. The phage is then propagated by growing on an appropriate bacterial culture. Moreover, phages can be lyophilized (vacuum dried) and directly used in capsules. In this form, the drug remains stable for 14 months at temperatures up to 55 °C.
Test: Viruses are non-cellular life forms
Time limit: 0
Navigation (job numbers only)
0 out of 10 tasks completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
Information
Instructions: “You are offered tasks with one or more correct answers out of five or more proposed.”
You have already taken the test before. You can't start it again.
The test is loading...
You must log in or register in order to begin the test.
You must complete the following tests to start this one:
results
Correct answers: 0 out of 10
Your time:
Time is over
You scored 0 out of 0 points (0)
| Average result |
| Your result |
Categories
- No category 0%
maximum of 15 points
| Place | Name | Recorded | Points | Result |
| Table is loading | ||||
| No data | ||||
Your result has been recorded in the leaderboard Loading
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- With answer
- With a viewing mark
- Task 1 of 10
1.
Non-cellular life forms
Right
Wrong
- Task 2 of 10
2.
First discovered viruses
Right
Wrong
- Task 3 of 10
3.
Virus that infects bacteria
Right
Wrong
- Task 4 of 10
4.
D.I. Ivanovsky discovered the virus
Right
Wrong
- Task 5 of 10
5.
Outer shell of the virus
Right
Wrong
- Task 6 of 10
6.
Bacteriophage discovered
Right
Wrong
- Task 7 of 10
7.
First proposed a vaccine against smallpox
Right
Wrong
- Task 8 of 10
8.
Depending on their structure, viruses are divided into
Right
Wrong
- Task 9 of 10
9.
Viral diseases
Right
Wrong
- Task 10 out of 10