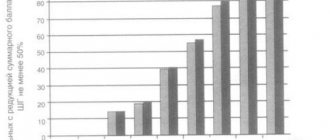
Treatment regimens for breast cancer patients in combination with chemotherapy
Day 1 – 30 minutes before chemotherapy TNF-T (Refnot®) at a dose of 200 thousand units once subcutaneously + chemotherapy + standard antiemetic therapy. 2nd, 3rd, 4th, 5th days - TNF-T (Refnot®) at a dose of 200 thousand units once subcutaneously 1 time per day. The course dose of TNF-T (Refnot®) is 1 million units (2 packs). (The effectiveness was proven in clinical studies conducted at the Federal State Institution Research Institute of Oncology named after N.N. Petrov under the guidance of the director of the institute, corresponding member of the Russian Academy of Medical Sciences, professor, MD V.F. Semiglazov).
Recommendations:
The drug is injected subcutaneously into the outer surface of the shoulder or thigh, the injection sites alternate. If hematological toxicity of treatment occurs, chemotherapy doses are reduced for all subsequent cycles in accordance with approved regimens. To enhance the antitumor and immunomodulatory effects of therapy, additional administration of INF gamma (Ingaron® produced by LLC NPP Pharmaklon, Russia) is recommended.
Regimen INF-gamma + TNF-T + chemotherapy:
The day before chemotherapy INF-gamma (Ingaron®) at a dose of 500 thousand IU once IM or SC. Day 1 – 30 minutes before chemotherapy TNF-T (Refnot®) at a dose of 200 thousand units once subcutaneously + chemotherapy + standard antiemetic therapy. 2nd, 4th, 6th, 8th days - INF gamma (Ingaron®) at a dose of 500 thousand IU once intramuscularly or subcutaneously once a day. Days 3, 5, 7, 9 – TNF-T (Refnot®) at a dose of 200 thousand units once subcutaneously once a day. Course dose of TNF-T (Refnot®) – 1 million units (2 packs); course dose of INF gamma (Ingaron®) – 2.5 million IU (1 package). (The effectiveness was proven in clinical studies conducted at the Federal State Institution Research Institute of Oncology named after N.N. Petrov under the guidance of the director of the institute, corresponding member of the Russian Academy of Medical Sciences, professor, MD V.F. Semiglazov).
Recommendations:
When administering drugs subcutaneously, the injection sites alternate. If hematological toxicity of treatment occurs, chemotherapy doses are reduced for all subsequent cycles in accordance with approved regimens.
Treatment regimens for cancer patients with REFNOT® as an immunomodulator
Chemotherapy regimen:
TNF-T (Refnot®) in a dose of 100 thousand to 400 thousand units once subcutaneously 5 days a week, then a 2-day break for 2-4 weeks before the course of chemotherapy. (The effectiveness has been proven in clinical studies conducted in the department of combined treatment methods of the State Institution "Russian Oncology Research Center named after N.N. Blokhin" RAMS under the leadership of the Deputy Director for Science, Corresponding Member of the Russian Academy of Sciences, Professor, Doctor of Medical Sciences M.R. Lichinitser).
Recommendations:
The drug is injected subcutaneously into the outer surface of the shoulder or thigh, the injection sites alternate. If thrombocytopenia is less than 80x103 / μl, the administration of TNF-T (Refnot®) should be stopped until the platelet count increases to at least 100x103 / μl. Treatment is carried out until progression or development of limiting side effects.
Monotherapy regimen:
TNF-T (Refnot®) in a dose of 100 thousand to 400 thousand units once subcutaneously 5 days a week, then a 2-day break for a long time (Efficacy has been proven in clinical studies conducted in the department of combined methods of treatment of GU "Russian Oncology Research Center named after N.N. Blokhin" RAMS under the leadership of Deputy Director for Science, Corresponding Member of the Russian Academy of Sciences, Professor, Doctor of Medical Sciences M.R. Lichinitser).
Recommendations:
The drug is injected subcutaneously into the outer surface of the shoulder or thigh, the injection sites alternate. If thrombocytopenia is less than 80x103 / μl, the administration of TNF-T (Refnot®) should be stopped until the platelet count increases to at least 100x103 / μl. Treatment should be carried out for a long time in the absence of progression, or until limiting side effects develop.
Thymosin alpha 1 – broad spectrum immunostimulant
Thymosin alpha 1 is an effective broad-spectrum immunostimulant and its place in modern therapy of infectious and oncological diseases
Advertising. Dietary supplement from Russian Peptide with fennel seed peptides – Endohels .
Promotes normalization of intestinal function and relaxation. Thymosin α1 (thymosin alpha 1, thymalfasin, Thymosin alpha 1, Tα1) is a peptide fragment derived from prothymosin alpha, a protein encoded by the PTMA gene in humans. Thymosin α1 was the first of the peptides contained in the 5th fraction of thymosin isolated from calf thymus tissue, which was completely sequenced in 1976, and then synthesized. A detectable concentration of thymosin α1 is also found in the blood serum of healthy individuals (~1 ng/ml). Unlike β-thymosins, thymosin α1 is produced from a longer precursor, prothymosin α, consisting of 113 amino acids, and is a sequence of 28 amino acids:
Ac -Ser-Asp-Ala-Ala-Val-Asp-Thr-Ser-Ser-Glu-Ile-Thr-Thr-Lys-Asp-Leu-Lys-Glu-Lys-Lys-Glu-Val-Val-Glu- Glu-Ala-Glu-Asn- OH
The N-terminal serine residue of the peptide is acetylated. The peptide is not glycosylated, has a molecular weight of 3108 Da and is characterized by high acidity.
The main feature of thymosins is their ability to participate in the regulation, differentiation and functioning of thymus-dependent lymphocytes (T cells, T lymphocytes). Thymosin α1 can induce T-lymphocyte differentiation and maturation, inhibit T-lymphocyte apoptosis, and stimulate the immune system to differentiate T-helper cells 1. Thymosin α1 is the main component of thymosin 5th fraction, and is considered responsible for the activity of this drug in restoring immune function in animals lacking a thymus gland. It is known to enhance cellular immunity in humans, as well as in experimental animals. The latter allows us to consider thymosin α1 a promising drug for enhancing the immune response in the treatment of various diseases.
Currently, thymosin α1 is approved as an immunomodulator in more than 37 countries, including Russia, for the treatment of hepatitis B and C, and is also used to enhance the immune response in the treatment of viral infections, immunodeficiencies, HIV/AIDS and malignant neoplasms. Thymosin α1 is most often used to treat chronic hepatitis B and as a vaccine adjuvant. Thus, clinical studies show that it may be useful in cystic fibrosis, septic shock, acute respiratory distress syndrome, peritonitis, acute cytomegalovirus infection, tuberculosis, acute respiratory syndrome and pulmonary infections, chronic hepatitis B and immunodeficiency states. In addition, the possibility of using thymosin α1 in the treatment of cancer, such as hepatocellular carcinoma, in combination with traditional chemotherapy has been studied. This approach is called immune anticancer therapy and is currently considered one of the most advanced treatment methods.
The mechanism of action of thymosin α1 as an immunostimulant is still not fully understood, but available data suggest that one of the ways to activate the immune response is to promote apoptosis of regulatory T cells (Tregs), which suppress the immune response. Their inhibitory effect helps reduce the damage caused by inflammation. Increasing the percentage of regulatory T cells is part of the mechanism of self-protection against severe inflammatory reactions, but may also cause secondary immune inhibition. Accordingly, a decrease in the proportion of regulatory T cells in the T-lymphocyte population promotes a more powerful immune response. One challenge is maintaining T cells at proper levels while maintaining a balance of pro- and anti-inflammatory responses.
Administration of thymosin α1 stabilizes fluctuations in the percentage of regulatory T cells and cytokine concentrations. In immunosuppressed hosts, thymosin α1 can inhibit the release of anti-inflammatory cytokines and stimulate the release of pro-inflammatory cytokines. At the same time, thymosin α1 suppresses the increase in the concentration of regulatory T cells, which is an important factor causing immunosuppression. Various in vitro studies have shown that CD4+, CD8+ and CD3+ cell counts are increased. Thymosin α1 has also been shown to increase the production of IFN-γ, IL-2, IL-3 and IL-2 receptor expression after activation by mitogens or antigens, increases the activity of NK cells, increases the production of migration inhibitory factor (MIF) and the antibody response to T -cellular antigens. Thymosin α1 was also found to prevent dexamethasone-induced apoptosis of thymocytes in vitro. In in vivo studies, administration of thymosin α1 to animals with immunosuppression caused by chemotherapy, tumor burden or radiation showed that the peptide protects against cytotoxic bone marrow damage, tumor progression and opportunistic infections, thereby increasing survival time and the number of survivors. Many of the effects of thymosin α1 in vitro and in vivo are interpreted as affecting either the differentiation of pluripotent stem cells into thymocytes or the activation of thymocytes into T cells. In vitro, this drug has also been shown to enhance the expression of Toll-like receptors (TLRs), including TLR2 and TLR9, in mouse and human dendritic cells, and also activates the NF-kB and JNK/P38/AP1 cascades. Activation of dendritic cells provides another possible pathway to explain the immunomodulatory and antiviral effects of thymalfasin.
In addition, thymosin α1 has been shown to restore monocyte expression of human leukocyte antigen (HLA-DR or mHLA-DR). The key advantage of using thymosin α1 in the clinic is its recognized biosafety with high in vivo tolerability without side effects over a wide dosage range. There are no known cases of intentional or accidental overdose in humans. Toxicological studies in animals have shown the absence of adverse reactions in single doses up to 20 mg/kg and in repeated doses up to 6 mg/kg/day for 13 weeks (the highest doses studied). The highest single dose tested in animals is equal to 800 times the clinical dose. Lack of toxicity was observed during clinical trials in more than 900 patients who received thymosin α1, which is in sharp contrast to most other biological response modifiers, such as interferon α (IFN-α) and interleukin-2 (IL-2), whose significant side effects and toxicity are problematic for most patients. The half-life of thymosin α1 is approximately 2 hours. In patients, within a day after administration, blood plasma levels return to the original level. Most (up to 60%) of thymosin α1 is excreted in the urine. With repeated administration, no accumulation of the drug was detected. These data indicate that the use of thymosin α1 fits well into the context of immunomodulatory therapy, as it is able to maintain immune homeostasis without toxic effects.
From 2002 to 2022 alone, at least 23 clinical trials of thymosin α1 involving several thousand patients are at various stages of completion; About 3 thousand scientific publications have been devoted to its study.
Some idea of the breadth of the spectrum of bioactivity of thymosin α1 is given by the list below, which is based on both experimental data and the results of calculations of potential targets of this drug in various diseases: cancer (colorectal, lung, breast, bladder, prostate, thyroid, stomach, leukemia, meningeal neoplasm, esophageal adenocarcinoma, lymphoma, hepatocellular carcinoma, neuroblastoma); autoimmune reactions (encephalomyelitis, autoimmune/multiple sclerosis, Crohn's disease; ulcerative colitis, systemic sclerosis, rheumatoid arthritis, lupus erythematosus, myasthenia gravis, scleroderma, lupus nephritis, secondary hyperparathyroidism, diabetes), a number of lung diseases, aging diseases and infectious diseases, and also about 45 other serious conditions. In addition, there is evidence for the use of thymosin α1 in cases of severe sepsis and for the prevention of invasive infections in bone marrow transplant patients. For clarity, the most important areas of applicability of thymosin α1 are presented in the diagram below:
| THYMOSIN α1 | |||||||||||||||
| Infectious diseases | Immunodeficiency and aging | Oncological diseases | |||||||||||||
| Hepatitis B Hepatitis C · AIDS · ARVI sepsis acute respiratory distress syndrome / COPD infections after bone marrow transplantation · severe sepsis and prevention of invasive infections during bone marrow transplantation | · aging autoimmune diseases · immunodeficiency vaccine adjuvant (vaccine enhancer) decreased bone mineral density · Alzheimer's disease · life extension | melanoma · lungs' cancer · hepatocellular carcinoma | |||||||||||||
Of the many studies conducted on the bioactivity of thymosin α1, let us consider the most practically significant results of clinical trials in accordance with the types of diseases. Since a huge number of studies have been conducted to date, the results of a meta-analysis are presented below.
Infectious diseases.
Chronic hepatitis B (CHB) remains a major clinical problem due to its extreme prevalence (more than 260 million people) and adverse consequences (liver cirrhosis, liver decompensation, and hepatocellular carcinoma - HCC). The primary goal of treatment for CHB is to permanently suppress viral replication and reduce liver inflammation, slowing or preventing decompensation, the development of cirrhosis, and other fatal consequences.
Clinical trials to evaluate the safety and efficacy of thymosin α1 were conducted for the treatment of patients with CHB. Thymosin α1 has been tested as monotherapy or in combination with currently available drugs, including IFN-α and nucleoside analogues. Overall, thymosin α1 resulted in disease remission rates ranging from 26 to 41% when administered subcutaneously for 6 to 12 months at a dose of 1.6 mg twice weekly in a sample of 56 patients. According to a meta-analysis that included two studies on two groups of patients (353 and 199 people, and the groups were comparable at baseline), a significant increase in virological response after therapy with thymosin α1 was shown at the end of treatment, 6 and 12 months after treatment: 0. 56, 1.67 and 2.67, respectively. It is reported that after 6 months of observation, significantly greater suppression of viral replication was observed with the use of thymosin α1 (OR = 3.71, 95% Cl: 2.05, 6.71, where OR is the odds ratio, Cl is the relative risk confidence interval), and alanine transaminase levels were normalized (OR = 3.12, 95% Cl: 1.74, 5.62) compared with patients receiving IFN-α alone. Immediately after treatment, there were no differences between the two groups in virological or biological response. However, patients receiving thymosin α1 were significantly more likely to demonstrate a complete response at 6 months at the end of follow-up (OR = 2.69, 95% Cl: 1.47, 4.91) compared with those receiving IFN-α. The only adverse event reported with thymosin α1 administration was discomfort at the injection site. Another meta-analysis, including 583 hepatitis B (HBeAg)-positive patients, compared the effect of lamivudine monotherapy with combination therapy of lamivudine plus thymosin α1. A statistically significant superiority of combination therapy compared with lamivudine monotherapy was revealed. Also, other studies have been conducted combining thymosin α1 with standard treatments for CHB (IFN-α and nucleoside analogues). In all cases, thymosin α1 increased the effectiveness of standard treatments without increasing their toxicity. Based on these data, thymosin α1 was selected as a therapeutic option for CHB by the Asia Pacific Association for the Study of the Liver.
Chronic hepatitis C is a global health problem (prevalence > 185 million people). Like hepatitis B, chronic hepatitis C (CHC) causes the development of cirrhosis and HCC.
A meta-analysis of a number of studies evaluating the activity of thymosin α1 for the treatment of CHC in combination with IFN-α showed that thymosin α1 in combination with IFN-α is more effective compared with IFN-α alone. Despite these promising results, the addition of thymosin α1 to retreatment of CHC patients (sample of 552) not responding to pegIFN alfa-2a or 2b plus ribavirin did not increase virologic response. However, it has been hypothesized that thymosin α1 may have a secondary therapeutic role as an adjuvant in preventing relapses in patients achieving a virological response during therapy.
Sepsis. Severe sepsis is associated with a high mortality rate of approximately 30%. In the United States, severe sepsis affects >750,000 people annually. Additionally, due to the emergence of carbapenem-resistant bacteria and the unavailability of effective antibiotics to treat this condition, there is a high need for a new treatment option. The main causes that play a decisive role in the fatal outcome of this disease are considered to be immunological disorders and the systemic inflammatory response, rather than infection per se, which is the rationale for the use of immunomodulatory agents such as thymosin α1.
Results from a large study conducted in China (361 patients with severe sepsis) showed that among patients receiving subcutaneous thymosin α1 at a dosage of 1.6 mg twice daily for 5 days, and then daily for an additional 2 days, 28 days after the start of treatment, mortality was 26.0% versus 35.0% in the control group, which received 1 ml of saline subcutaneously on the same schedule. The relative risk of death in the group receiving thymosin α1 was 0.74 compared with the control group. In another large clinical trial, thymosin α1 was used in combination with a protease inhibitor (342 patients with severe sepsis). 28-day and 90-day mortality were examined and the rates were significantly better in the thymosin α1 group compared with control (25.1 vs. 38.3% and 37.1 vs. 52.1%, respectively). A meta-analysis of the effectiveness of thymosin α1-based immunomodulatory therapy in severe sepsis, summarizing 12 controlled clinical trials in 1480 patients, 751 of whom received thymosin α1, showed significantly lower mortality, regardless of cause, in the group of patients receiving thymosin α1 (average hazard ratio 0. 68).
Chronic obstructive pulmonary disease. A clinical trial in 108 elderly COPD inpatients showed a reduction in the incidence of secondary infections and a shorter hospital stay for the group of patients treated with thymosin α1. No side effects were observed. In another study, 80 patients with COPD received 10 doses of thymosin α1 or standard therapy. At 3 and 6 months after treatment, the number of patients with exacerbation of symptoms was significantly lower in the group receiving thymosin α1 treatment.
Acute respiratory failure is a life-threatening condition with a mortality rate of up to 40%. In a study of 46 kidney transplant patients, mortality was significantly lower in the thymosin α1 group than in the control group (21.9 vs. 50%).
Thymosin α1 as a vaccine adjuvant (vaccine enhancer ).
As part of the normal aging process, there is a gradual decline in hormone production and functioning of the thymus gland, which can contribute to a decline in immunity. It has been established that thymosin α1 is a TLR9 agonist. TLRs are a family of proteins that mediate innate immunity; agonist stimulation of one or more TLRs can enhance the adaptive immune response, which is critical for humoral immunity. Thymosin α1 causes activation and stimulation of signaling pathways that initiate the production of immune-related cytokines, and also affects T cells, which is described in more detail in the part devoted to the mechanism of action of the drug.
In studies on the effectiveness of vaccination against the background of administration of thymosin α1, the comparison object was the monovalent H1N1 influenza vaccine with an MF59 adjuvant. The response to high dose thymosin α1 was statistically significant and was seen earlier than with MF59 and was maintained at 42 days post-vaccination. The tolerability of this combination was excellent.
HIV AIDS . Since immune responses play an important role in preventing the acquisition of HIV infection in humans, it is believed that the induction of a strong immune response, especially the killer T cell (CTL) response against HIV-1, may be of great importance in preventing the occurrence of acquired immunodeficiency syndrome (AIDS). A study using a combination of thymosin α1, zidovudine and IFN-α showed that such therapy leads to a significant increase in the number and activity of mature CD4+ T cells and a decrease in HIV titer. Another interesting study looked at the safety and effectiveness of thymosin α1 in combination with highly active antiretroviral therapy (HAART) to stimulate immune reconstitution. The results showed that thymosin α1 was well tolerated and could significantly increase T receptor excision ring (sjTREC) levels. The latter is an important criterion for immunological monitoring. The long-term therapeutic use of thymosin α1 in HIV is still under investigation.
Oncological diseases . The first clinical studies were performed in the late 90s on patients with melanoma and non-small cell lung cancer, and showed a positive effect when treated with a combination of thymosin α1 with dacarbazine in combination with IL-2 or with IFN-α in patients with metastatic melanoma. A study in 20 subjects with unresectable stage III or IV metastatic melanoma showed good tolerability, prolonged mean survival time and mean time to progression, with an overall response rate of 50% and 15% of patients over 3 years. The study also showed that combining thymosin α1 with IFN-α resulted in significant protection against dacarbazine-induced reduction in NK cell activity and CD4+ T cell counts. This result was reliably confirmed in a sample of 488 patients with metastatic melanoma. Other experimental studies have shown that the addition of thymosin α1 together with IFN-α to cisplatin/etoposide or ifosfamide chemotherapy in patients with non-small cell lung cancer resulted in improved immune parameters and response to therapy. Chemotherapy toxicity was also significantly reduced, with a decrease in the percentage of subjects with stage III or IV myelosuppression (0 vs. 50% of controls). The use of thymosin α1 in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma (HCC) resulted in a statistically significant increase in survival (82 versus 41%) and a significant increase in the immune response (cytotoxic T cells and NK cells) in samples ranging from 12 to 57 people. As shown by many years of research and clinical practice, thymosin α1, in combination with standard therapy, affects many types of cancer. The effects of thymosin α1 on HCC, non-small cell lung cancer and melanoma support the drug as a useful agent for the treatment of these diseases. Also, in vivo and in vitro studies have shown an association with breast cancer, suggesting a potential improvement in response when combined with chemotherapy. An in vitro study on breast cancer cells demonstrated the ability of thymosin α1 to induce apoptosis through activation of the mitochondrial apoptotic cascade and PTEN (phosphatase and tensin homolog), which mediates inhibition of the PI3K/Akt/mTOR signaling pathway. It is emphasized that treatment with thymosin α1 enhances immune responses in peripheral blood mononuclear cells, and the drug can also restore immune surveillance weakened by a tumor or chemotherapy/radiotherapy, accelerating the differentiation of T cells and increasing the activity of natural killer cells, the expression of the class I antigen of the major histocompatibility complex ( MHC class I) and tumor-associated antigens on target cells. Thymosin α1 has been demonstrated to enhance the cytotoxicity of iNKT cells (a small population of T cells expressing the invariant Vα24-Jα18 T cell receptor with significant potential for anticancer therapy) against colon cancer by enhancing CD1d expression. Thymosin α1 has also been proposed as an effective adjuvant for dendritic cell vaccine therapy in the treatment of hematological malignancies such as acute lymphoblastic leukemia by promoting the phenotypic and functional maturation of dendritic cells, which have the ability to induce antitumor immunity to leukemic cells.
In general, innovative discoveries in the treatment of cancer are due to the powerful synergism of thymosin α1 and chemotherapy, which is based on the dual effect of the drug on both effector cells and tumor cells.
Currently, thymosin α1 is marketed under the trade name ZADAXIN (thymalfasin) as a lyophilisate containing 1.6 mg of chemically synthesized thymosin α1, identical to human, 50 mg of mannitol and supplied with sodium phosphate buffer to adjust the solution to pH 6.8. The drug is intended for subcutaneous administration and is prescribed for monotherapy or in combination with interferon for the treatment of chronic hepatitis B and C, and as an adjuvant for vaccination against influenza and hepatitis B. The recommended dosage is 1.6 mg (900 µg/m2) twice a week for over a period of 6 to 12 months. For patients weighing up to 40 kg, a dosage of 40 µg/kg is recommended. Side effects are rare and minor: discomfort at the injection site, rare cases of erythema, transient muscle atrophy, polyarthralgia combined with swelling of the arms and rash.
The emergence of new drugs and biological products often means the development of new treatment options. “Repurposing” known drugs by identifying the possibility of a previously unknown application of an existing drug on other cellular targets or diseases can reduce time, cost and risk by reducing or eliminating several preclinical and clinical testing steps required for de novo drug discovery and development. In this regard, despite its almost 50 years of fame, thymosin α1 is still the object of increased interest as a potential drug with low toxicity for the treatment of a wide range of diseases.
Thymosin alpha 1 (3 mg)
Thymosins
TB-500 – the ubiquitous healing peptide
Thymosins and Thymosin alpha 1
Thymus preparations: Timulin, Timalin, Timogen, Imunofan
https://russianpeptide.com/timozin-beta-4-i-ego-aktin-svyazyivayushhiy-domen-stimulyatsiya-rosta-volos/
Endohels. What is this?