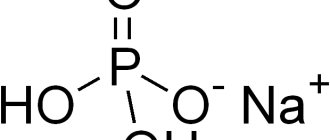
Sodium sulfate is an inorganic compound, a salt of sodium and sulfuric acid H2SO4. In the literature it may be called sodium sulfate, anhydrous sodium sulfate, natural sodium sulfate, anhydrous reussin, anhydrous sodium sulfate, food additive E514. Formula Na2SO4.
The reagent occurs in nature in anhydrous form (mineral thenardite), as a crystalline hydrate (mirabilite or Glauber's salt) and is part of double salts (glauberite with calcium sulfate, astrachanite and vanthoffite with magnesium sulfate). There is a lot of it in salt lakes of the chloride-sulfate type, in the Kara-Bogaz-Gol gulf of the Caspian Sea.
Sodium sulfate is obtained from natural mineral salts (thenardite, mirabillite), bottom sediments of salt lakes or as a by-product of metallurgical production; enterprises producing hydrochloric acid, chromium compounds, artificial fibers.
| Chemical protective gloves KShchS-2 | Sodium sulfate anhydrous |
For whom is it intended and for whom is it contraindicated?
The drug is used to suppress the reaction to poisoning:
- cyanide;
- hydrocyanic acid;
- lead;
- cadmium;
- mercury;
- arsenic;
- lithium;
- aluminum, etc.
Sodium thiosulfate is used in the treatment of lupus erythematosus, neuralgia, arthritis, allergies, and scabies.
The only contraindication for medical procedures is individual intolerance to its component composition. During treatment, side effects may occur in the form of allergies or discomfort in the area where the medication was administered.
Receipt
Natural springs
Sodium sulfate occurs in the form of the mineral thenardite, and the crystalline hydrate Na2SO4.10H2O occurs in the form of mirabilite or Glauber's salt.
Laboratory methods of obtaining
Sodium sulfate is prepared by reacting sulfuric acid with sodium hydroxide or carbonate:
H2SO4 + 2NaOH = Na2SO4 + 2H2O;
H2SO4 + Na2CO3 =Na2SO4 + H2O + CO2↑.
Production of sodium sulfate in industry
In industry, sodium sulfate was prepared from sodium chloride and sulfuric acid:
H2SO4 + 2NaCl =Na2SO4 + 2HCl↑,
but now this does not make sense - natural sources are enough.
Features of sodium thiosulfate therapy
The instructions recommend the following methods and dosages:
- Topical application is used for scabies mites. A 60% solution is rubbed into the skin of the entire body. After it dries, additional treatment is carried out with a 6% solution of hydrochloric acid.
- Intravenous administration is used to cleanse the body of poisonous and toxic substances. 30% of the drug is administered intravenously, 5-50 ml. The exact dosage depends on the severity of the disease and the type of toxic compound.
- Oral – for procedures a 10% composition is used, a single dosage is equal to 2-3 g of medication.
In gynecological practice, the drug is part of complex therapy:
- endocrine infertility;
- ovarian cysts;
- genital tuberculosis.
If necessary, sodium thiosulfate is included in microenemas. This approach is used to detect adhesions in the pelvic area and inflammation of the reproductive tract. A 10% solution is suitable for enemas; 30 to 50 doses of medication are sufficient for one procedure.
Treatment for psoriasis involves taking medication orally. Cleansing the body has a positive effect on the immune system and facilitates the course of many chronic diseases. Therapy for psoriasis continues for 5-12 days; you can take from 10 to 20 ml of the product per day. The exact dosage depends on the weight and tolerance of the component composition.
A measured amount of medicine is diluted in half a glass of water and consumed before going to bed. If the medicine provokes a laxative effect, then the dose is reduced to 10 ml per day.
Sodium thiosulfate can be used to cleanse the circulatory and lymphatic systems. The procedures can improve the condition of nail plates and hair, get rid of allergies, depression, and improve general condition.
Cleansing procedures last 10 days; half an ampoule can be used at one time; the solution is diluted in half a glass of water. The first procedure is designed for the morning - half an hour before breakfast, the second - an hour before dinner.
SODIUM SULPHATE. Natrium sulfas.Synonyms: sodium sulfate, Glauber's salt.
Properties.
It is colorless, transparent, air-weathering crystals with a bitter-salty taste. Easily soluble in water (in cold 1:3 and 3:1 at a temperature of 33°C), insoluble in alcohol. Contains about 50% water of crystallization. When heated, it melts in its water of crystallization. Incompatible with lime, barium, lead, and mercury salts.
Release form. Available in powder form.
Store in a well-closed container.
Action and application.
Sodium sulfate in small doses irritates the receptors of the stomach and intestines, increasing secretion, revitalizes motor skills and improves digestion, and has an anti-catarrhal effect.
In large doses, the drug helps to increase osmotic pressure in the intestines, where large amounts of water accumulate, which dilutes the chyme and reflexively enhances peristalsis. This is also facilitated by irritation of the intestinal mucosal receptors by the drug solution. When using salt in a concentrated solution, dehydration occurs. The laxative effect occurs after 8-14 hours and manifests itself throughout the intestines.
Sodium sulfate also stimulates bile formation and peristalsis of the excretory tract, which promotes the excretion of metabolic products with bile.
When used externally, hypertonic solutions of sodium sulfate promote cleansing of the wound surface and rapid healing of wounds.
Sodium sulfate is prescribed orally as a laxative with plenty of water for intestinal blockage and colitis, overfeeding, flatulence, deworming and poisoning with various substances; as a distracting and dehydrating agent for inflammation of the brain, pulmonary congestion and pleurisy, peritonitis, arthritis, dropsy and rheumatic inflammation of the hooves in horses (with a small amount of water).
Together with herbal bitters and sodium chloride, the drug is prescribed to improve appetite and digestion, for catarrh of the stomach and intestines; for liver disease, it is used as a choleretic and a means of treating and preventing gallstone disease.
In the form of a 1% sodium sulfate solution, it is used for hypotension and atony of the proventriculus by washing the rumen. Used as an antidote for poisoning with barium, mercury, and lead salts.
Sodium sulfate is prescribed orally
animals in the following doses (g): as a laxative - horses 300-500; cattle 400-800, deer 100-300, sheep 50-100, pigs 25-50, dogs 10-25, cats 3-10, chickens 2-4, foxes and arctic foxes 5-20, minks 5-15; as a choleretic agent - horses 150-250, cattle 250-600, dogs 10-15; to improve digestion - horses and cattle 25-50, sheep 5-12 g, pigs 3-5 g, dogs 0.2-0.5 g.
The dose of anhydrous sodium sulfate is 2 times less.
In recommended doses, sodium sulfate does not cause complications and has no side effects.
Manufacturer's instructions
The instructions draw attention to the following nuances:
- combination with iodides and bromides leads to their lack of effectiveness;
- combination with drugs that cause rhodanization weakens their effectiveness;
- immediate administration of sodium thiosulfate is necessary in case of cyanide intoxication; delay causes death;
- The powder of the drug has a specific smell, reminiscent of the aroma of sodium acetate - you can check the authenticity of the product with iodine, a chemical reaction will lead to its discoloration.
Therapeutic measures for poisoning require constant supervision by the attending physician. If symptoms of intoxication resume after their initial disappearance, it is necessary to repeat the procedure with half the dose.
Properties of sodium sulfate
Physical properties
| Property | Description |
| Appearance | White crystalline substance |
| Molar mass | 142.04 g/mol |
| Density | 2.68 g/cm3 |
| Melting temperature | 883°C |
| Standard molar enthalpy of formation at 298K ΔH°298, kJ/mol | −1387.9 (t)2 |
| Standard molar entropy at 298 K S°298, J/(mol•K) | 149.58 (t) |
| Standard molar heat capacity at 298 K Cp298, J/(mol•K) | 127,3 |
| Solubility in water, g/100 g | at 0°C - 4.5 at 20°C - 19.2 at 32.4°C - 49.8 at 100°C - 42.3 |
Chemical properties
When dissolved in water, sodium sulfate dissociates into ions:
Na2SO4↔ 2Na++ SO42-.
The solution has a neutral reaction.
When interacting with acids, it forms acid salts:
Na2SO4 + HСl = NaHSO4 + NaCl.
Enters into exchange reactions to form insoluble sulfates:
Na2SO4 + CaCl2 = 2NaCl + CaSO4↓,
Na2SO4 + SrCl2 = 2NaCl + SrSO4↓,
Na2SO4 + BaCl2 = 2NaCl + BaSO4↓.
Analogs
Sodium thiosulfate can cause reactions in the body. The appearance of side effects or signs of intolerance to the medication requires its replacement. The list of popular analogues is presented:
- Algisorb, Amyl nitrite, Braidan;
- Glation, Dipiroxime, Carboxim;
- Lobelin, Naloxone, Sodium nitrite;
- Pelixim, Pentacin, Unithiol;
- Ferrocin, Amber-antitox.
Independent replacement of medication is strictly prohibited. The selection of an analogue is carried out by the attending physician, based on diagnostic data and the reasons that necessitated the need to change the therapeutic course.
pharmachologic effect
The drug has chondrostimulating, regenerating, anti-inflammatory and analgesic effects. Participates in the construction of the basic substance of cartilage and bone tissue. Has chondroprotective properties; enhances metabolic processes in hyaline and fibrous cartilage and subchondral bone; inhibits the activity of enzymes that cause degradation (destruction) of articular cartilage; stimulates the production of proteoglycans by chondrocytes; influences phosphorus-calcium metabolism in cartilage tissue, stimulates its regeneration, and participates in the construction of the basic substance of bone and cartilage tissue. It has anti-inflammatory and analgesic properties, helps reduce the release of inflammatory mediators and pain factors into the synovial fluid through synoviocytes and macrophages of the synovial membrane, and suppresses the secretion of lykotriene and prostaglandins. The drug prevents connective tissue degeneration and reduces calcium loss, accelerating bone tissue restoration processes.
Chondroitin sulfate slows the progression of osteoarthritis and osteochondrosis. Promotes the restoration of the joint capsule and cartilaginous surfaces of the joints, prevents the collapse of connective tissue, and normalizes the production of joint fluid.
The clinical effect is manifested by an improvement in joint mobility and a decrease in pain intensity, while the therapeutic effect persists for a long time after the end of the course of therapy. When treating degenerative changes in joints accompanied by secondary synovitis, the effect is observed within 2-3 weeks from the start of the course.
Possessing structural similarity to heparin, it can potentially prevent the formation of fibrin thrombi in the synovial and subchondral microcircular bed.
Combination with alcohol
Sodium thiosulfate is actively used in the treatment of alcohol dependence. Treatment involves taking medication and alcohol at the same time to cause certain symptoms:
- attacks of vomiting with nausea;
- hand trembling;
- increased sweating;
- disturbances in heart rate;
- cough, etc.
This approach allows us to develop an aversion to ethanol. Therapeutic procedures take 16-20 days, take place every day, then three times a week and once a month. The drug solution is administered intravenously, the dosage is selected for each patient separately.
Application
• Included in household detergents and cleaning products, laundry powders. • In glass manufacturing plants. • In the textile industry and leather industry. • At pulp and paper mills for the production of paper using the sulphate method. • In non-ferrous metallurgy, chemical industry, oil production and oil refining. • In the construction industry - an additive to concrete to accelerate setting and increase frost resistance. • In laboratory practice as a cheap and convenient desiccant org. solvents. • In pharmacology and veterinary medicine - in the preparation of laxatives and solutions for nasal rinsing. • Antidote for heavy metal poisoning (forms insoluble salts with them, easily removed from the body). • Limited use in the food industry as additive E514 (acidity regulator, emulsifier, stabilizer, filler for low-calorie products). Used as a desiccant for spices, vegetables, mushrooms, and meat products. • In the cosmetic industry it is part of anti-cellulite bath salts.
Side effect
When using the drug in persons with hypersensitivity to the drug, the following disorders are possible:
- from the immune system: allergic reactions, angioedema;
- from the skin and subcutaneous tissue: skin rash, itching, erythema, urticaria, dermatitis;
- from the gastrointestinal tract: dyspepsia.
Redness, itching, and hemorrhages are possible at the injection site.
Overdose
Currently, cases of drug overdose have not been described.
Contraindications
- hypersensitivity to the components of the drug;
- bleeding and tendency to bleeding, thrombophlebitis;
- children under 18 years of age, pregnancy and breastfeeding (there are no data on the safety of the drug).
Carefully
Simultaneous use of the drug with direct-acting anticoagulants.
Use during pregnancy and breastfeeding
The use of Chondroitin sulfate during pregnancy is contraindicated. If the drug is used during breastfeeding, breastfeeding should be discontinued.