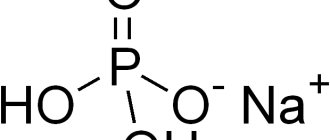
The substance is supplied in the form of a white crystalline powder with a hemostatic effect. A water-soluble synthetic analogue of vitamin K3 increases blood clotting. Vitamin K promotes blood clotting by enhancing the synthesis of coagulation factors II, VII, IX, and X in the liver. Vitamin K deficiency can cause capillary bleeding.
Prothrombin (factor II) in the blood in the absence of Ca2+ and thromboplastin and in the presence of proconvertin (factor VII), factors IX, X is converted into thrombin. Under the influence of thrombin, fibrinogen transforms into fibrin, which is the basis of a thrombus (blood clot). Substrate stimulation of the production of K-vitamin reductase occurs, the action of which is aimed at the production of vitamin K. The drug takes part in the hepatic synthesis of K-vitamin-dependent plasma hemostasis factors. Menadione sodium bisulfite acts as a pharmaceutical substance for veterinary drugs.
Indications
Indications for use are hypoprothrombinemia (treatment and prevention) due to the development of vitamin K deficiency, obstructive jaundice, dysfunction of the pancreas, small intestine, prolonged diarrhea, celiac disease, etc.
Menadione sodium bisulfite is used for the treatment and prevention of diseases such as hypovitaminosis and disorders of protein metabolism in decorative and songbirds, pigeons during molting, stress, vaccination, ovariitis, salpingitis. It is prescribed to increase egg production, feed palatability, improve the safety of chicks, and restore feather cover. Side effects may include hyperbilirubinemia, jaundice, and dizziness. The effect of indirect anticoagulants is weakened.
Contraindication for use is increased individual sensitivity to the components of the drug. Ineffective for Werlhof's disease and hemophilia.
Buy Vikasol FST tablets 15 mg No. 20 in pharmacies
Tradename:
Vikasol
International nonproprietary name:
menadione sodium bisulfite
Dosage form:
pills.
Description:
tablets are white or almost white, flat-cylindrical in shape with a bevel.
Compound:
Active substance: vikasol (menadione sodium bisulfite) – 15 mg.
Excipients: sugar, potato starch, sodium pyrosulfite, silicone emulsion, talc, stearic acid.
Pharmacotherapeutic group:
vitamin
Pharmacological properties
Pharmacodynamics
A synthetic water-soluble analogue of vitamin K, promotes the synthesis of prothrombin and proconvertin, increases blood clotting by enhancing the synthesis of coagulation factors II, VII, IX, X.
It has a hemostatic effect (vitamin K deficiency causes increased bleeding).
In the blood, prothrombin (factor II) in the presence of thromboplastin and calcium ions, with the participation of proconvertin (factor VII), factors IX (Christmas factor), X (Stewart-Prower factor) transforms into thrombin, under the influence of which fibrinogen is converted into fibrin, which constitutes the basis of a blood clot (thrombus). Substrate stimulates vitamin K reductase, which activates vitamin K and ensures its participation in the hepatic synthesis of vitamin K-dependent plasma hemostasis factors.
The onset of the effect is 7-12 hours after oral administration.
Pharmacokinetics
After intramuscular administration, it is easily and quickly absorbed. Accumulates in tissues in small quantities. After going through a cycle of metabolic activation, it is oxidized in the liver to the diol form. It is excreted by the kidneys (up to 70%) and with bile almost exclusively in the form of metabolites. High concentrations of vitamin K in feces are due to its synthesis by intestinal microflora.
Indications for use
Hemorrhagic syndrome associated with hypoprothrombinemia, hypovitaminosis K (including obstructive jaundice, hepatitis, liver cirrhosis, prolonged diarrhea).
Bleeding after wounds, trauma and surgical interventions; as part of complex therapy for dysfunctional uterine bleeding, menorrhagia.
Hypoprothrombinemia due to a decrease in the content of factors II, VII, IX, X while taking certain medications (coumarin and indanedione derivatives, salicylates, some antibiotics).
Contraindications
Hypersensitivity to the components of the drug. Increased blood clotting, thromboembolism.
Carefully
- glucose-6-phosphate dehydrogenase deficiency, liver failure, pregnancy.
For obstructive jaundice, preference should be given to the injection form of the drug.
Directions for use and doses
Vikasol is prescribed orally for 3-4 days, after a four-day break the course is repeated. The daily dose can be divided into 2-3 doses. The maximum single dose for adults is 30 mg, the maximum daily dose for adults is 60 mg.
For surgical interventions with possible severe parenchymal bleeding, it is prescribed for 2-3 days before surgery. In pediatrics: from 3 to 5 years - 7.5 mg/day; from 5 to 14 years – 15 mg/day; over 14 years old - 30 mg/day.
Side effect
Allergic reactions: facial flushing, skin rash (including erythematous, urticaria), skin itching, bronchospasm.
From the blood system: hemolytic anemia, hemolysis in newborns with congenital deficiency of glucose-6-phosphate dehydrogenase.
Other: hyperbilirubinemia, jaundice. Rarely – dizziness, transient decrease in blood pressure, “profuse” sweat, tachycardia, “weak” pulse filling, changes in taste sensations.
Overdose
In case of overdose, hypervitaminosis K occurs, manifested by hyperprothrombinemia, hyperbilirubinemia, and rarely, children may have convulsions.
Interaction with other drugs
Reduces and blocks the anticoagulant effect of neodicoumarin and phenylin. Does not affect the anticoagulant activity of heparin.
Antacids reduce absorption due to the precipitation of bile salts in the initial part of the small intestine.
Simultaneous administration with broad-spectrum antibiotics, quinidine, quinine, salicylates in high doses, antibacterial sulfonamides requires an increase in the dose of vitamin K.
Cholestyramine, colestipol, mineral oils, sucralfate, dactinomycin reduce the absorption of vitamin K, which requires increasing its dose.
special instructions
For hemophilia, von Willebrand's disease and Werlhof's disease, the drug is ineffective.
For diseases leading to impaired bile outflow, parenteral administration is recommended.
Prophylactic administration of vitamin K in the third trimester of pregnancy is ineffective due to the low permeability of the placenta to it.
Release form
Tablets of 10 or 20 pieces in a blister pack. 1, 2 or 3 blister packs in a cardboard pack along with instructions for use.
Storage conditions
List B. In a dry place, protected from light and out of reach of children.
Best before date
3 years. Do not use after the expiration date.
Conditions for dispensing from pharmacies
On prescription
Medications
In the Russian Federation, as of 05.2020, 3 medicinal preparations of vitamin K3 (menadione sodium bisulfite) were registered in different dosage forms:
- RADOSTIN® Antistress
- (Produced by LLC AVZ S-P, 141305, Sergiev Posad) - a combined drug in the form of a solution for oral use based on retinol, thiamine, riboflavin, cyanocobalamin, ascorbic acid, cholecalciferol, tocopherol, biotin, menadione sodium bisulfite, methionine. This vitamin complex for birds is prescribed for the prevention of hypovitaminosis and protein metabolism disorders in decorative and songbirds and pigeons during the period of vaccination, molting, and stress. Can be used during oviposition; - Healthyvit
- (manufactured by Healthyway LLC, Moscow) - a combined preparation based on vitamins A, D3, E, K, B1, B2, B6, B12, PP, B5, B9, H, B4, B8 in the form of a solution for injections. Compensates for the deficiency of biologically active substances in the body of animals that occurs during stress, preventive vaccinations and deworming, after illnesses, and helps to normalize metabolism in animals. "Helsivit" is prescribed for therapeutic and preventive purposes in cattle and small cattle, pigs, dogs and cats for various types of hypovitaminosis, as well as for weakened immunity, infection, during the rehabilitation period after various diseases, during pregnancy and as an adjuvant treatment during antibiotic therapy.
Recommended storage conditions for the drugs are in sealed manufacturer's packaging, in a place protected from light, at a temperature of no more than 25℃. More accurate information is provided on the packaging of drug manufacturers.
In pharmaceuticals, analogues of menadione sodium bisulfite with the same pharmacological effect are sold in the form of the following drugs:
- Vitamin K1 – phylloquinone.
- Vitamin K2 – menaquinone.
- Vitamin K4 –2-methyl-1,4-naphthohydroquinone.
- Vitamin K5 –2-methyl-4-amino-1-naphthohydroquinone.
- Vitamin K6 – 2-methyl-1,4-diaminonaphthoquinone.
- Vitamin K7 –3-methyl-4-amino-1-naphthohydroquinone.
Sold in the following dosage forms: solutions for intramuscular administration, tablets, powders, solutions for oral administration.
Packaging of E222 supplement
According to international standards, sodium hydrosulfite can be packaged and transported in two types of containers. The first type includes steel barrels placed in plywood barrels. Another type of packaging is plastic bags placed in steel barrels with a wall thickness of more than half a millimeter. This ensures maximum tightness of the container and prevents any attempts to contact the substance with the surrounding air. As mentioned above, it only takes a small amount of water to cause a dangerous reaction. Sodium hydrosulfite is packaged in 50 kilograms per container. These measures are prescribed in all regulations of countries that manufacture, supply or use the E222 additive.
Doses and method of administration
RADOSTIN® Antistress
For medicinal purposes, it is administered to birds in a dose of 3-8 drops in the feed daily for 5 days. For parrots and large birds, the drug is administered with food in a dose of 25 drops. After 10 days, the course is repeated. For the purpose of prevention, vitamin K3 is added to bird feed once a month in the amount of 3 drops per head. Parrots and large individuals are given 25 drops for 3-5 days. No overdose symptoms were found.
Healthyvit
for prophylaxis, animals are administered intramuscularly or subcutaneously once every 3 weeks, for medicinal purposes - once every 7-15 days in the following doses:
- Cattle - 5-6 ml per head;
- Horses - 3-5 ml per head;
- Calves and foals - 2-3 ml per head;
- Goats and sheep - 1-2 ml per head;
- Lambs and kids – 1 ml per head;
- Adult pigs - 3-5 ml per head;
- Replacement young pigs – 2 ml per head;
- Weaned piglets - 1.5 ml per head;
- Suckling piglets – 1 ml per head;
- Newborn piglets - 0.5 ml per head;
- Dogs weighing over 15 kg - 0.5 ml per head;
- Dogs weighing up to 15 kg - 0.2 ml per head;
- Puppies up to 3 months of age - 0.1 ml per head;
- Cats - 0.1 ml per head.
To improve reproductive functions, "Helsivit" is prescribed to females twice, the first time - 1-2 weeks before the expected date of insemination and the second time - 1.5-3 months before the expected date of birth. Course duration is 2-3 injections. The treatment course can be re-prescribed, if necessary, after 30 days.
Properties of sodium hydrosulfite
In addition to the properties of a preservative and antioxidant, sodium hydrosulfite has the properties of a stabilizer and bleach. Externally, it is a yellow or white crystalline mass or a powder with a powerful odor of sulfur dioxide. This substance is quite unstable: in combination with water, pyrosulfite is formed. Sodium hydrosulfite dissolves in water, but this is not observed in alcohols, fats and oils.
The synthesis of sodium hydrosulfite is carried out by combining sodium carbonate and sulfur dioxide. The substance produced in this way is a fire hazard if it comes into contact with water in an air space with a high oxygen content. At the same time, in its pure form it does not pose any danger. Upon contact with water, sulfur is released and ignites. The human body can consume up to 0.7 milligrams of E222 per day per kilogram of body weight.
Content:
- Properties of sodium hydrosulfite
- Packaging of E222 supplement
- Main producers of sodium hydrosulfite
- Application of sodium hydrosulfite
- The benefits and harms of sodium hydrosulfite for the human body
3. METHODS OF ANALYSIS
3.1. A point sample from the tank is taken with a special sampler, the diagram of which is presented in GOST 2184, or with a sampler of a similar design, ensuring the collection of a representative sample over the entire height of the tank. The volume of a spot sample must be at least 1 dm3. Samples are taken from the barrels using a pipette or siphon. The volume of a spot sample must be at least 0.5 dm3. (Changed edition, Amendment No. 2).
3.2. (Deleted, Amendment No. 2).
3.3. The selected point samples are combined together, thoroughly mixed and a combined sample with a volume of at least 0.5 dm3 is taken.
3.4. Place the combined sodium bisulfite sample in a clean, dry, tightly sealed glass jar. A label is placed on the jar indicating the name of the product, the name of the manufacturer, the batch number and the date of sampling.
3.5. Defining Appearance
3.5.1. Equipment Funnel V-56-80 HS according to GOST 25336. Cylinder 1(3)-100-2 according to GOST 1770.
3.5.2. Carrying out the analysis The appearance of technical sodium bisulfite is determined visually at room temperature. The sample is considered to have passed the test if sodium bisulfite placed in a cylinder against a background of white paper meets the requirements set out in paragraph 1 of the table.
3.5-3.5.2. (Changed edition, Amendment No. 3).
3.6. Determination of the mass fraction of sodium bisulfite in terms of SO2
3.6.1. Equipment, reagents and solutions General purpose laboratory scales according to GOST 24104, 2nd accuracy class with the highest weighing limit of 200 g; The use of other scales is permitted, the technical and metrological characteristics of which correspond to those specified in the standard. Weighing glass SV-14/8 according to GOST 25336. Measuring flasks 2-500-2 according to GOST 1770. Pipettes with a capacity of 10 and 50 cm3. Burette with a capacity of 50 cm3. Conical flasks with a capacity of 500 cm3 in accordance with GOST 25336. The use of imported utensils is allowed, the technical and metrological characteristics of which correspond to those specified in the standards. Distilled water according to GOST 6709, free of carbon dioxide; prepared according to GOST 4517. Iodine according to GOST 4159, solution concentration c
(1/2J2)=0.1 mol/dm3 (0.1 N);
prepared according to GOST 25794.2. Sulfuric acid according to GOST 4204, solution concentration c
(1/2H2SO4) = 1 mol/dm3 (0.1 N);
prepared according to GOST 25794.2. Soluble starch according to GOST 10163, solution with a mass fraction of 0.5%; prepared according to GOST 4919.1. Sodium sulphate (sodium thiosulfate) according to GOST 27068, solution concentration c
(Na2S2O3·5H2O) = 0.1 mol/dm3 (0.1 N); prepared according to GOST 25794.2. (Changed edition, Amendment No. 2, 3).
3.6.2. Carrying out analysis
5 g of sodium bisulfite is weighed in a weighing cup. The weighing result in grams is recorded accurate to the fourth decimal place. The sample is quantitatively transferred into a 500 cm3 volumetric flask, adjusted to the mark with water and mixed. In a conical flask with a capacity of 500 cm3, place 50 cm3 of water, 50 cm3 of iodine solution, 10 cm3 of sulfuric acid solution and add 50 cm3 of sodium bisulfite solution with a pipette. Excess iodine is titrated with a solution of sodium sulfate until a light yellow color appears, then 2 cm3 of starch solution is added and titration is continued until the blue color of the solution disappears. At the same time, prepare a control solution with the same amounts of reagents, but without the analyzed product.
3.6.3. Processing of results Mass fraction of sodium bisulfite in terms of SO2 ( X
) as a percentage are calculated by the formula where
V
is the volume of sodium sulphate solution with a molar concentration of exactly 0.1 mol/dm3 (0.1 N), used for titration of the control solution, cm3:
V1
is the volume of sodium sulphate solution with a concentration of exactly 0.1 mol /dm3, spent on titration of excess iodine, cm3;
0.003203 - the amount of sulfur dioxide (SO2), corresponding to 1 cm3 of iodine solution of concentration exactly c
(1/2J2) = 0.1 mol/dm3 (0.1 N), g;
m
—weight of sample, g;
X1
is the mass fraction of sulfur dioxide (SO2), determined according to clause 3.7.3, %.
The result of the analysis is taken as the arithmetic mean of two parallel determinations, the absolute discrepancy between which does not exceed the permissible discrepancy of 0.5%. When receiving an analysis result in the ranges of 24.0-24.2 and 25.3-25.5% (for a 1st grade product) and 22.5-22.7% (for a 2nd grade product), it is necessary to carry out two additional definitions. In this case, the arithmetic mean of the results of four parallel determinations is taken as the result of the analysis, the absolute discrepancy between the most different values of which does not exceed the permissible discrepancy equal to 0.7%. The permissible total absolute error of the analysis result is ±0.35% with a confidence level of P
= 0.95.
3.6.2, 3.6.3. (Changed edition, Amendment No. 1, 2, 3).
3.7. Determination of the mass fraction of sodium sulfite in terms of SO2
3.7.1. Equipment, reagents and solutions General purpose laboratory scales according to GOST 24104, 2nd accuracy class with the highest weighing limit of 200 g; It is permitted to use other scales whose technical and metrological characteristics correspond to those specified in the standard. Weighing cups SV-14/8 according to GOST 25336. Measuring flasks 2-500-2 according to GOST 1770. Pipettes with a capacity of 10 and 50 cm3. Burette with a capacity of 50 cm3. Conical flask Kn-1-500 THS in accordance with GOST 25336 with a ground stopper. It is allowed to use imported utensils whose technical and metrological characteristics correspond to those specified in the standards. Distilled water according to GOST 6709, free of carbon dioxide; prepared according to GOST 4517. Mixed indicator (methyl red and methylene blue), prepared according to GOST 4919.1. Hydrochloric acid according to GOST 3118, solution concentration c
(HCl) = 0.1 mol/dm3 (0.1 N);
prepared according to GOST 25794.1. Sodium hydroxide (sodium hydroxide) according to GOST 4328, solution concentration c
(NaOH) = 0.1 mol/dm3 (0.1 N); prepared according to GOST 25794.1. Formalin according to GOST 1625, solution 3:7; prepared as follows: 300 cm3 of formaldehyde is mixed with 700 cm3 of water and neutralized with a solution of sodium hydroxide in the presence of a mixed indicator until a grayish-green color appears in the solution. (Changed edition, Amendment No. 2, 3).
3.7.2. Carrying out analysis
6 g of sodium bisulfite is weighed in a weighing cup. The weighing result in grams is recorded accurate to the second decimal place. The sample is transferred quantitatively into a conical flask with a capacity of 250-300 cm3, into which 20 cm3 of formaldehyde solution is first placed. Then add 80 cm3 of water, 10 drops of indicator and titrate with a solution of hydrochloric acid until the green color of the solution changes to red-violet. If, after adding the indicator, the solution turns red-violet, then sodium sulfite is considered to be absent. (Changed edition, Amendment No. 2).
3.7.3. Processing of results Mass fraction of sodium sulfite in terms of SO2 ( X1
) as a percentage is calculated by the formula where
V
is the volume of hydrochloric acid solution with a concentration of exactly 0.1 mol/dm3, consumed for titration, cm3;
0.0064 - the amount of sulfur dioxide (SO2), corresponding to 1 cm3 of hydrochloric acid solution with a concentration of exactly 0.1 mol/dm3, g; m
is the mass of the sample, g. The result of the analysis is taken as the arithmetic mean of the results of two parallel determinations, the absolute discrepancy between which does not exceed the permissible discrepancy of 0.2%.
The permissible absolute total error of the analysis result is ±0.1% with a confidence level of P
= 0.95. (Changed edition, Amendment No. 2,3).
3.8. Determination of the mass fraction of iron in terms of FeO
3.8.1. Equipment, reagents and solutions General purpose laboratory scales according to GOST 24104, 2nd accuracy class with the highest weighing limit of 200 g; The use of other scales is permitted, the technical and metrological characteristics of which correspond to those specified in the standard. Volumetric flasks 2-100-2 according to GOST 1770. Pipettes with a capacity of 10 and 50 cm3. Droppers 2-50 HS GOST 25336. It is allowed to use imported utensils whose technical and metrological characteristics correspond to those specified in the standard. Photoelectric colorimeters KFK-2 or similar. Distilled water according to GOST 6709. Red Congo indicator paper. Hydroxylamine hydrochloride according to GOST 5456, solution with a mass fraction of 10%. 2,2′-Dipyridyl, solution with a mass fraction of 0.5%; prepared as follows: 0.5 g of the drug is dissolved in 95 cm3 of hot water with the addition of 5 cm3 of hydrochloric acid solution of concentration c
(HCl) = 0.01 mol/dm3.
Ferroammonium alum [iron (III)-ammonium sulfate], a solution containing 1 mg of iron (Fe3+) per 1 cm3 of solution; prepared according to GOST 4212. 10 cm3 of the prepared solution is diluted with a solution of hydrochloric acid with a concentration of 0.01 mol/dm3 to 1 dm3; 1 cm3 of the resulting solution corresponds to 0.01 mg of iron - solution A, solution A is used only on the day of preparation. Hydrochloric acid according to GOST 3118, solution 1:1 and solution concentration c
(HCl) = 0.01 mol/dm3 (0.01 N); prepared according to GOST 25794.1. Sodium acetate according to GOST 199, solution with a mass fraction of 20%.
O-phenanthroline, solution with a mass fraction of 0.2%; Prepare as follows: 0.5 g of the drug is dissolved in 250 cm of hot water. The solution is stored in a dark glass bottle.
3.8.2. Construction of a calibration graph To construct a calibration graph, comparison solutions are prepared: into volumetric flasks with a capacity of 100 cm3, 0.5 is taken from the burette, respectively; 1.0; 2.0; 3.0; 4.0; 5.0; 6.0 cm3 of solution A. The volume of solutions is adjusted to 20 cm3 with water. 4 cm3 of a solution of hydroxylamine hydrochloride, 4 cm3 of a solution of sodium acetate and 4 cm3 of a solution of o-phenanthroline or 2,2′-dipyridyl are successively poured into each flask. After adding each reagent, the contents of the flasks are mixed. Fill the volume of the solution with water to the mark and mix again. The resulting reference solutions contain, respectively, 0.005; 0.010; 0.020; 0.030; 0.040; 0.050; 0.060 mg iron. At the same time, a control solution is prepared, into which all the same reagents are added, except for solution A. The optical density of the resulting solutions is measured on a photoelectric colorimeter (on the left drum) in relation to the control solution in cuvettes with a light-absorbing layer thickness of 50 mm, using a green filter (=490 -540 nm). Based on the data obtained, a calibration graph is constructed, plotting the amount of iron contained in the comparison solutions in milligrams on the abscissa axis, and the corresponding optical densities values on the ordinate axis.
3.8.1, 3.8.2. (Changed edition, Amendment No. 2, 3).
3.8.3. Carrying out analysis
50 cm3 of the solution obtained according to clause 3.6.2 is placed in a volumetric flask with a capacity of 100 cm3. A 1:1 solution of hydrochloric acid is added dropwise to the solution until the indicator paper turns blue-violet. Then 4 cm3 of a solution of hydroxylamine hydrochloride, 4 cm3 of a solution of sodium acetate and 4 cm3 of a solution of o-phenanthroline or 2,2′-dipyridyl are successively added. Fill the volume of the solution with water to the mark and mix. At the same time, a control experiment is carried out with the same amounts of reagents, but without the analyzed solution. The optical density of the analyzed solution is measured in relation to the corresponding aliquot of the control solution, as when constructing a calibration graph. The iron content in the analyzed sample in milligrams is determined using a calibration curve. (Changed edition, Amendment No. 3).
3.8.4. Processing of results Mass fraction of iron in terms of FeO ( X2
) as a percentage is calculated by the formula where
a
is the iron content found from the calibration curve, mg;
1.2865 — conversion factor from Fe to FeO; m
— weight of the sample, g. The result of the analysis is taken as the arithmetic mean of the results of two parallel determinations, the absolute discrepancy between which does not exceed the permissible discrepancy equal to 0.002%.
The permissible absolute total error of the analysis result is ±0.001% with a confidence level of P
= 0.95. (Changed edition, Amendment No. 2, 3).
3.9. Determination of the mass fraction of unbound sulfur dioxide
3.9.1. Equipment, reagents and solutions Pipettes with a capacity of 1 cm3. Conical flask Kn-1-500 THS in accordance with GOST 25336 with a ground stopper. Cylinders 2-25-2 in accordance with GOST 1770. Droppers 2-50 HS in accordance with GOST 25336. It is allowed to use imported utensils whose technical and metrological characteristics correspond to those specified in the standard. Distilled water according to GOST 6709. Methyl orange (indicator); prepared according to GOST 4919.1. (Changed edition, Amendment No. 2, 3).
3.9.2. Carrying out analysis
Place 1 cm3 of sodium bisulfite in a conical flask with a capacity of 50 cm3, add 10 cm3 of distilled water and add 2-3 drops of methyl orange solution. The product is considered to comply with the requirements of this standard if, upon addition of methyl orange, a red color of the solution is not observed.
1a. SAFETY REQUIREMENTS
1a.1. Sodium bisulfite is fire- and explosion-proof and toxic. The toxicity of sodium bisulfite is determined by the possibility of releasing sulfur dioxide (SO) from the solution. Sulfur dioxide (SO2) is a colorless gas with a pungent odor and is irritating to mucous membranes. Long-term exposure causes acute inflammation of the mucous membranes and bronchi, resulting in coughing, sore throat and chest, and lacrimation. The maximum permissible concentration of SO2 in the air of the working area of industrial premises is 10 mg/m3 (according to GOST 12.1.005).
1a.2. Production premises and laboratories in which work with sodium bisulfite is carried out must be provided with supply and exhaust ventilation in accordance with GOST 12.4.021 and sanitary facilities. Production equipment and communications in places where gases may form must be equipped with local suction, ensuring the air condition of the working area in accordance with the requirements of GOST 12.1.005. The concentration of harmful substances is monitored using methods developed in accordance with GOST 12.1.016.
1a.3. Those working with sodium bisulfite must be provided with special clothing in accordance with GOST 12.4.016 and standards approved in accordance with the established procedure, as well as personal respiratory protection equipment - respirators of the U-2K, ShB-1 “Petal” type in accordance with GOST 12.4.028.
1a.1-1a.3. (Changed edition, Amendment No. 3).
1a.4. When producing, using, transporting and storing sodium bisulfite, it is necessary to comply with the requirements of GOST 17.2.3.02 and safety rules for the production of the basic chemical industry.
1a.5. Waste disposal is carried out in accordance with sanitary rules for the accumulation, transportation, neutralization and disposal of industrial waste.
1a.4, 1a.5. (Introduced additionally, Amendment No. 3).
Application of sodium hydrosulfite
Additive E222 is now widely used in the food industry due to its antioxidant and preservative properties. At food industry enterprises it is added to products, in particular to canned fruits. Main purpose: fight against germs. Sodium hydrosulfite is also necessary in modern wine production (especially dry wines). Additive E222 allows you to preserve the taste and stop the oxidation processes caused by vinegar. Of course, some wine producers, in order to preserve the product, prefer not to add sodium hydrosulfite, but then the drink may acquire an unhealthy brownish tint. Naturally, in this case the taste will change.
The process of fighting microbes is as follows: sodium hydrosulfite comes into contact with water and fermentation products. Thus, sulfur dioxide is formed, in which bacteria, fungi and yeast die.
Sodium hydrosulfite is an additive known, perhaps, to all confectioners in Ukraine and Russia. E222 is added to jelly and ice cream, marmalade and jams. But not just sweets. The presence of sodium hydrosulfite can also be seen on the labels of packages of dried fruits, meat, sausages, fish products, canned and fresh-frozen vegetables and fruits. E222 is an almost indispensable substance for the production of semi-finished products based on mushrooms and potatoes, as well as liquid pectins.
The E222 additive is also used in other industries. In particular, in the production of fabrics and dyeing of cotton, sodium hydrosulfite removes active dyes. Sodium hydrosulfite is a good reducing agent for various dyes, as well as a preservative for bleaching fabrics. This substance is also used in heavier industry. For example, in biochemical engineering it is used to maintain the absence of air in the reactor. Sodium hydrosulfite is used in the light and chemical industries.