Antianginal drugs are a group of drugs that are used to treat coronary heart disease - angina and myocardial infarction.
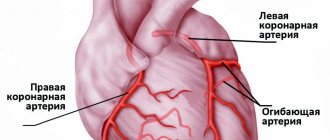
Coronary heart disease (CHD) is a pathological condition associated with oxygen starvation of the heart muscle (myocardium) due to insufficient blood supply to the vessels of the heart.
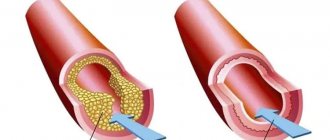
Chronic ischemic heart disease is manifested by angina pectoris. The causes of angina are usually narrowing of the lumen of the coronary (heart) vessels by atherosclerotic plaque (angina pectoris) or vasospasm (vasospastic angina).
Acute coronary heart disease is manifested by unstable angina and myocardial infarction. The cause of acute ischemic heart disease is partial or complete blockage of the lumen of blood vessels by a thrombus.
Unstable angina is considered a pre-infarction condition. Most often, it turns into an acute myocardial infarction, but if quickly recognized and treated, unstable angina can resolve quite safely.
The narrowing of the lumen of the coronary vessels leads to a decrease in the delivery of blood (and with blood, oxygen and nutrients) to the myocardium, which is manifested by oxygen starvation of the heart muscle, pain during physical activity or psycho-emotional stress.
Complete blockage of the lumen of the coronary vessels leads to a sharp lack of oxygen in the areas of the heart fed by these vessels and, in the absence of treatment, their death - necrosis.
Medicines used for angina are called antianginal drugs (from the old Latin name for angina: angina pectoris - angina pectoris).
Classification of antianginal drugs
Depending on the mechanism of action, antianginal drugs are classified into:
- drugs that increase oxygen delivery to the heart: menthol, validol (menthol in isovaleric acid menthyl ester), dipyridamole;
- drugs that reduce the heart's need for oxygen: β-blockers (propranolol, metoprolol, atenolol, betaxolol, bisoprolol, nebivolol, carvedilol);
- bradycardic drugs (ivabradine);
- organic nitrates and nitrate-like agents - sydnonimines (nitroglycerin, isosorbide mononitrate, isosorbide dinitrate, pentaerythrityl tetranitrate, molsidomine);
Antihypoxants. What are they and who needs them?
Antihypoxants are drugs that improve the body's absorption of oxygen and reduce the need of organs and tissues for oxygen, thereby increasing the body's resistance to oxygen deficiency.
Research convincingly shows that pharmacological agents that affect mitochondrial complexes are the most promising in the fight against hypoxia in sports.
Conventionally, antihypoxants can be divided into groups:
– drugs with direct antihypoxic action; – correcting metabolism in the cell: – membrane-protective action, – direct energizing effect (affecting the redox potential of the cell, the Krebs cycle and mitochondrial respiratory chain complexes); – acting on the transport function of the blood: – increasing the oxygen capacity of the blood, – increasing the affinity of hemoglobin for oxygen, – vasoactive substances of endogenous and exogenous nature.
*Hypoxene
Olifen (hypoxene). Antihypoxant. The mechanism of action of polyphene on cells is to reduce oxygen consumption by tissues and to use it more economically under hypoxic conditions.
Olifen is an enzyme of the respiratory chain of synthetic nature. Possessing a high electron exchange capacity due to the polyphenolic structure of the molecule, olyphen has a shunting effect at the stage of formation of lactic acid from pyruvic acid, forming acetyl Co A, which is then involved in the tricarboxylic acid cycle.
Olifen at the molecular level facilitates tissue respiration under hypoxic conditions due to the ability to directly transfer reduced equivalents to enzyme systems. The drug repeatedly compensates for the lack of ubiquinone under hypoxic conditions, as it contains a large number of functional centers.
Thus, olyphen compensates for the activity of the mitochondrial respiratory chain in the presence of damage in its areas.
The antioxidant effect of olifene is associated with its polyphenolic structure, which protects cell and mitochondrial membranes from the destructive effects of free radicals formed during lipid peroxidation. This pathological process is triggered by extreme physical and psycho-emotional impacts on the body.
Olifen improves hypoxia tolerance by increasing the rate of oxygen consumption by mitochondria and increasing the coupling of oxidative phosphorylation.
Being a direct-acting drug, it can provide oxygen to any cell due to the small size of its own molecules. In this regard, its use is possible for all types of hypoxia.
Economical consumption of energy reserves occurs due to the transfer from glycolytic to aerobic oxidation of energy substrates, i.e. to a more favorable metabolic mechanism.
In this case, the energy output increases 19 times, since anaerobic glycolysis of one molecule produces 2 ATP molecules, and aerobic glycolysis produces 38 ATP molecules.
A water-soluble antioxidant, having a high energy capacity, sets a large number of electron traps. The redox potential of olyphene is 680, that of coenzyme Q10 is 122.
Indications for use in sports: increasing performance when performing muscular work in extreme competition conditions; economical consumption of oxygen by tissues under hypoxic conditions; prevention and overcoming the state of chronic fatigue; accelerating the recovery of the body after undergoing stress; improvement of peripheral blood flow.
It is eliminated from the body after 6-8 hours. There are practically no side effects. In rare cases, nausea and dry mouth are possible.
Olifen improves the absorption of other substances (medicines, vitamins) by 25%.
*Coenzyme Q-10
Ubiquinone (coenzyme Q-10, coenzyme Q10) is a substance that is produced by the body and comes with food. It is found in beef (especially in internal organs - heart, liver, kidneys), fatty fish, spinach, peanuts and whole grains. Although coenzyme Q10 (CoQ-10) can be found in many fresh foods, it is unstable and is easily destroyed by oxidation during processing and cooking.
CoQ-10 is involved in the electron transport respiratory chain of mitochondria. Reduces tissue damage caused by hypoxia, generates energy and increases exercise tolerance. As an antioxidant, it slows down the aging process (neutralizes free radicals by donating their electrons). Strengthens the immune system.
Our body can produce CoQ-10 if it receives vitamins B2, B3, B6, C, folic and pantothenic acids in the required quantities. If any of these vitamins are deficient, CoQ-10 synthesis is inhibited.
Has no toxic doses or side effects. CoQ-Yu is taken in a dosage of 30 to 100 mg per day. At the moment there is not enough objective data and reliable extensive research on CoQ-1.
*Nicotinamide
Nicotinamide. Nicotinic acid amide and nicotinic acid itself (vitamin PP, niacin, vitamin B3), being a prosthetic group of the enzymes NAD and NADP and hydrogen carriers, are involved in the processes of tissue respiration, the metabolism of fats, carbohydrates, and amino acids.
*Cytochrome C
Cytochrome C (cyto Mac). Hemoprotein, catalyst of cellular respiration. Stimulates oxidative reactions and thereby activates metabolic processes in tissues, reduces tissue hypoxia in various pathological conditions. The effect occurs a few minutes after intravenous administration and lasts for several hours.
Allergic reactions are possible during use. For those predisposed to allergic reactions, it is recommended to carry out a test with the introduction of 0.5-1 ml of cytochrome C, diluted 1:10; or 0.1 ml intradermally.
*Reamberin
Reamberin. The solution (1.5%) for infusion is a well-balanced polyionic solution with the addition of succinic acid, containing: sodium chloride 6.0 g, potassium chloride 0.3 g, magnesium chloride 0.12 g, sodium succinic acid 15 g, water for injection up to 1 liter. A balanced drug with an osmolarity close to the normal osmolarity of human blood plasma.
The main pharmacological effect of the drug is due to the ability to enhance compensatory activation of aerobic glycolysis, reduce the degree of inhibition of oxidative processes in the Krebs cycle, in the mitochondrial respiratory chain with an increase in the intracellular fund of high-energy compounds (ATP and creatine phosphate). Sodium succinate (succinic acid) according to the clinical classification belongs to substrate antihypoxants. Incorporated into energy metabolism as a substrate, succinic acid salts direct oxidation processes along the most economical path.
Reamberin has a hepatoprotective effect, reducing the duration of lipid peroxidation processes and preventing the depletion of glycogen reserves in liver cells.
The maximum level of drug concentration in the blood during intravenous administration is observed in the first minute after administration. After 40 minutes, its concentration returns to values close to background.
*Riboxin
Inosine (riboxin). The action of inosine is antihypoxic, antiarrhythmic, anabolic. Increases the activity of a number of Krebs cycle enzymes and energy balance. It has a positive effect on metabolic processes in the myocardium - it increases the force of contractions and promotes more complete relaxation of the myocardium in diastole (binds calcium ions that entered the cytoplasm at the time of cell excitation), resulting in an increase in stroke volume; blood supply to tissues improves, including coronary circulation.
Used for the prevention of metabolic disorders in the myocardium during extreme physical exertion, for myocardial dystrophy due to heavy physical activity, heart rhythm disturbances, and for the prevention of liver diseases.
When used, tachycardia, exacerbation of gout, hyperemia and itching of the skin, and other allergic reactions are possible.
*Actovegin
Actovegin (solcoseryl). A drug of biological origin. Activates cellular metabolism by increasing the transport and accumulation of glucose and oxygen, enhancing intracellular utilization. Improves trophism and stimulates the regeneration process.
*Mildronate
Mildronate. Improves metabolic processes. Increases performance, reduces symptoms of mental and physical stress; has cardioprotective and regulating effects on the cellular immune system; eliminates functional disorders in the somatic and autonomic nervous systems. The drug causes a decrease in the content of free carnitine and reduces carnitine-dependent oxidation of fatty acids.
Bioavailability – 78%. Maximum plasma concentrations are achieved 1–2 hours after administration. The half-life is 3-6 hours.
Used for recovery after training and competitive load; physical overstrain, overtraining.
In rare cases, skin itching, dyspeptic symptoms, tachycardia, agitation, and changes in blood pressure are possible.
Use cautiously for tachycardia and hypotension.
*Vinpocetine
Cavinton (vinpocetine). A drug that improves cerebral circulation and metabolic processes in brain tissue; promotes the transport of oxygen to tissues due to a decrease in the affinity of red blood cells for it, enhancing the absorption and metabolism of glucose; reduces increased blood viscosity, improves microcirculation. Glucose metabolism switches to the energetically more favorable aerobic direction. It also stimulates anaerobic glucose metabolism.
Prescribed in cases of acute and chronic cerebral circulatory failure (transient ischemia in endurance sports); post-traumatic and hypertensive encephalopathy (traumatic sports); to reduce memory impairment; for dizziness; headache; movement disorders.
*Vitamins C and E, adaptogens, nootropics, lithium hydroxybutyrate, citric and fumaric acids also have an antihypoxic effect.
With the combined use of antihypoxants, their effect is enhanced.
Basics of treatment of coronary heart disease
Antianginal drugs are used both to relieve (eliminate) an attack of angina, and to prevent the occurrence of attacks systematically.
In addition to antianginal drugs, in the treatment of stable and vasospastic angina, drugs for the treatment of atherosclerosis (antiatherosclerotic) and the prevention of thrombus formation (antiplatelet agents) are used.
For the treatment of unstable angina and acute myocardial infarction, fibrinolytics (drugs that dissolve blood clots) and anticoagulants and antiplatelet agents (drugs that prevent recurrent thrombus formation) are used primarily not with antianginal drugs.
Recent decades have been characterized by significant advances in the prevention and treatment of cardiovascular diseases (CVD): arterial hypertension (AH); various forms of coronary heart disease (CHD) – acute coronary syndrome (ACS), myocardial infarction (MI); chronic heart failure (CHF). These achievements are due to the introduction into clinical practice of modern high-tech methods for diagnosing and treating left ventricular failure, which are based on a clear understanding of the mechanisms of development of ischemia and death of cardiomyocytes (structural and functional units of the myocardium), and adaptive changes in central hemodynamics.
IHD is widespread throughout the world, especially in economically developed countries, and occupies a leading place in the structure of disability and mortality from CVD among a socially significant age group of the population. In most European countries, the prevalence of IHD is 20–40 thousand per 1 million population. Mortality from diseases of the circulatory system in the Russian Federation, according to medical statistics for 2010, amounted to 56.5% of total mortality; Of these, more than half account for ischemic heart disease as the cause of death.
IHD as an “independent disease” was identified by the World Health Organization (WHO) only in 1965 due to the increasing frequency of this pathology, its dominant participation in the progression of cardiac pumping disorders in CHF, and was included in the International Statistical Classification of Diseases, Injuries and Causes of Death.
In IHD, there is a discrepancy between the level of oxygen consumption by the myocardium and the volume of its delivery by the coronary bloodstream. Adequate energy supply for the pumping activity of the heart in a wide range of its activity - from rest to the level of maximum load (corresponding to the level of basal metabolism of the whole organism) depends on the state of the coronary reserve. Coronary reserve is the ability of the coronary vascular bed to increase coronary blood flow many times due to dilatation of the coronary vessels adequately to the oxygen needs of the myocardium.
Oxygen is a key component of oxidative phosphorylation in the synthesis of ATP, the “fuel” that ensures the functioning of cardiomyocytes and the pumping activity of the heart in general. Energy metabolism in the myocardium represents interconnected mechanisms of O2 delivery and its utilization by the subcellular structures of the cardiomyocyte - mitochondria [1, 2, 4].
To provide energy for its activity, the heart “utilizes” various biological substrates: carbohydrates (glucose, glycogen, lactate), free fatty acids (FFA), and, to a lesser extent, amino acids (proteins). Regardless of the energy substrate, in the final stage of the breakdown of biological substrates, acetyl coenzyme A is formed, which enters the tricarboxylic acid cycle (Krebs cycle), and with the participation of O2 in the mitochondria, the energy substrate ATP is formed.
Under physiological conditions, 10% of ATP is formed during oxidative phosphorylation in mitochondria due to aerobic glycolysis (the breakdown of glucose to pyruvate). The amount of ATP generated as a result of aerobic glycolysis is not enough to ensure the operation of ion channels of the sarcolemma, in particular for the calcium pump of the sarcoplasmic reticulum (SR), which consumes up to 50% of the synthesized energy to ensure diastolic relaxation. Replenishment of the remaining amount of phosphate energy for the functioning of the cardiomyocyte as a whole, with normal oxygen supply, occurs due to the oxidation of FFA. The metabolism of FAs during oxidative phosphorylation provides up to 80% of ATP synthesis. However, FFA oxidation, compared to glycolysis, is a less efficient source of ATP: “fuel” for the heart pump. When oxidizing FFAs, the production of the same amount of ATP requires approximately 10% more oxygen than during glycolysis [1, 4].
FFAs penetrate into mitochondria through active transport, for which the carnitine palmitine enzyme complex is responsible, then β-oxidation of FFAs occurs in mitochondria. This process is strictly controlled and depends mainly on the intensity of FFA translocation into mitochondria. In the case of moderate ischemia, aerobic oxidation of FFA and glucose decreases and anaerobic glycolysis becomes the main source of ATP. Under these conditions, glycogen reserves are mobilized to support glycolysis.
With the development of varying degrees of ischemia (partial or complete occlusion of the coronary artery), anaerobic glycolysis remains the only source of limited ATP formation. As O2 delivery decreases, the activity of oxidative metabolism decreases, producing a limited amount of ATP. A pronounced imbalance between the oxygen demand during the oxidation of glucose and FFA towards the latter leads to the fact that during ischemia in the mitochondria of cardiomyocytes, ATP synthesis switches to β-oxidation of FA with the accumulation of many under-oxidized active forms of FA acyl-coenzyme-A (Acyl-CoA) and acylcarnitine (AcCar). ), which further exacerbates the uncoupling of oxidative phosphorylation (Fig. 1). Underoxidized active forms of FA, in particular AcCar and Acyl-CoA as metabolites, block the transport of ATP from the site of synthesis in mitochondria to the site of their intracellular consumption, have a destructive effect on the membrane - the sarcolemma, increasing the energy deficit necessary for the life of cardiomyocytes [2, 4, 6, 10].
In parallel, under conditions of severe ischemia (lack of blood flow), lactate and H+ accumulate in cardiomyocytes, i.e., against the background of anaerobic metabolism, protons (H+, Na+) accumulate and “acidification” of the cytoplasm occurs. H+ and Na+ ions are exchanged for other cations (mainly Ca2+), as a result of which the cardiomyocytes are “overloaded” with Ca with the formation of incomplete diastole - myocardial contracture (Fig. 2).
Modern advances in the study of cell function (in particular, endothelium) of various organs indicate the key role of oxidative stress - excessive formation of reactive oxygen species (ROS - O2) in the formation of CVD through lipid peroxidation (LPO) of the cell membrane. The main source of ROS in cells is mitochondria, during the normal functioning of which 98% of the supplied oxygen is used for the oxidation of substrates with the formation of ATP (the main energy substrate of cells) and 2% for the synthesis of ROS, which can increase significantly in various pathological conditions (Fig. 3) .
A decrease or cessation of O2 delivery to the heart muscle can be caused by various mechanisms: from spasm to total blockage of the coronary artery. After restoration of coronary blood flow, damaged mitochondria are not able to completely utilize the “surging” supply of oxygen, part of which is used by other oxidative systems of cells and is accompanied by the formation of an increased amount of ROS. The activity of one of the powerful oxidative enzymes, xanthine oxidase, is at a low level under conditions of aerobic metabolism, but increases sharply under hypoxia, in addition, with the conversion of Fe3+ to Fe2+. The combination of these two factors contributes to the excessive formation of ROS [8]. Excessive formation and release of free radicals (ROS) activate lipid peroxidation (LPO) with damage to cell membranes, which consist of phospholipids, cholesterol and protein inclusions that act as ion channels or receptors.
All of the above is an incentive for clinicians in the treatment and prevention of possible complications in the conditions of ischemic episodes in various regions (heart, central nervous system): the use of drugs with antioxidant and antihypoxic pharmacological orientation, which have pleiotropic effects (cardio-, neurocytoprotection), restoration of aerobic intracellular metabolism. A typical representative of drugs with similar pharmacokinetic and pharmacodynamic properties is Actovegina.
Actovegin is a highly purified hemodialysate from the blood of calves, obtained by ultrafiltration, does not contain endotoxins and antigens and consists of biologically active physiological components with high biological activity: amino acids, oligopeptides, nucleosides, products of carbohydrate and fat metabolism. The pharmacological composition of Actovegin is formed by 2-stage ultrafiltration using filters to isolate molecules of different sizes. The molecular weight of the final filtered product does not exceed 5000 Daltons. The composition of Actovegin was tested using modern analytical techniques, including gas liquid chromatography combined with mass spectrometry. Data from quantitative methods for analyzing possible metabolites showed that Actovegin is a combination of more than 200 bioactive molecules [6, 7, 10].
The atomic emission spectrometry method showed the presence in Actovegin of macro-electrolytes (Mg, Na, Ca, P, K) and microelements (Si, Cu), which are included in the prostatic groups of antioxidant enzymes (superoxide dismutase, glutathione peroxidase, catalase). The antioxidant effect of Actovegin is due to superoxide dismutase activity [8]. Magnesium, which is included in Actovegin, is a component of cardiopeptide fragments and enzymes and functions as a catalytic center that provides control and launch of enzymobiochemical intracellular processes.
The anti-ischemic effect of Actovegin at the cellular level is carried out due to the transfer of cell energy metabolism towards aerobic glycolysis with inhibition of β-oxidation of fatty acids. Actovegin®, selectively inhibiting 3-ketaocetyl-CoA catalase, slows down the ß-oxidation of fatty acids, while competitively restoring the coupling between glycolysis and oxidative decarboxylation, which overall leads to an increase in the amount of ATP, which underlies the anti-ischemic protection of cardiomyocytes by Actovegin (Fig. 4) .
Experimental studies at the cellular level have shown that Actovegin® supports the energy metabolism of the heart. The cardioprotective effect of Actovegin is due to its ability to maintain the physiological level of creatine phosphate (the main carrier of energy inside the cell) and ATP under conditions of ischemia, stabilize the pH inside the cell (prevents the development of intracellular metabolic acidosis), and reduce damage to the membrane - sarcolemma - by lipid peroxidation caused by free radicals. Normalization of metabolic balance leads to limiting the accumulation of inorganic phosphate, Na and Ca inside the cell while maintaining normal K concentration. At the same time, Actovegin® reduces the level of migration and infiltration of polynuclear neutrophils (inhibition of chemotaxis) in ischemic and reperfused heart tissues, which reduces autoimmune damage to the myocardium without causing influence on central hemodynamics [4, 5, 9].
Oxidative stress causes breakage of 1 strand of DNA, which leads to activation of the nuclear enzyme poly-ADP-ribose polymerase (PARP). Excessive activation of PARP has negative consequences in the form of triggering successive cellular processes that ultimately stop glycolysis and the process of mitochondrial respiration (oxidative phosphorylation - Krebs cycle), which leads to cell death due to energy depletion and activation of oxidative stress [8].
Further studies have confirmed the role of PARP metabolism as an important mechanism in the development of endothelial dysfunction in cardiovascular pathologies caused by impaired carbohydrate metabolism - diabetes, and it has recently been confirmed that PARP may be involved in the development of diabetic polyneuropathy. Summarizing these data, we can make an assumption about the important role of PARP in ischemic heart disease, cerebrovascular diseases and diabetes [3, 5, 7, 8].
MW Elmlinger et al., using brain cell cultures (primary hippocampal neurons), in their study showed the inhibitory effect of Actovegin on oxidative stress processes. In neurons treated with increasing concentrations of tert-butyl hydroperoxide (> 0.2 mM), an increase in intracellular ROS levels was found (p < 0.001), but in the case of Actovegin in cultured neurons, a dose-dependent decrease in the severity of oxidative stress was noted after 10 days (p < 0.001 at concentrations > 0.3 µg/ml) [8]. In in vivo studies, the effect of Actovegin on the analyzed parameters in experimental diabetic polyneuropathy corresponded to the results obtained in this in vitro study [6, 7].
Actovegin® has a multifaceted effect by normalizing the consumption and use of oxygen, increasing the entry of glucose into cells, thereby restoring cellular metabolism [6, 9, 10]. Actovegin® enhances oxidative processes, shifting the balance of redox reactions towards oxidation, which helps to increase the content of high-energy phosphates, such as ATP and creatine phosphate. K. Schwabe showed that Actovegin® activates intracellular oxidative processes and accelerates not only energy, but also reserve metabolism, which in the case of heart disease is accompanied by increased accumulation of glycogen and potassium. These data were one of the first observations showing a direct positive effect of the drug Actovegin® on the metabolism of the brain and myocardium [5, 8].
Previous studies document that Actovegin® has an insulin-like effect through the activation of GLUT 1–4, stimulating cellular metabolism, increasing oxygen consumption and energy production. One of the constituent parts of Actovegin fractions is Inositol-Phosphate-Oligosaccharide (IFO-fraction), which, through the activation of cAMP and adenylate cyclase, promotes intracellular glucose utilization, stimulates the efficiency of O2 consumption and reduces the formation of lactates.
The effect of Actovegin on glucose transport into the cardiomyocyte is insulin-independent (does not affect insulin receptors), since it is realized through the direct activation of GLUT 1–4, so its effect persists even against the background of insulin resistance in patients with type 2 diabetes. At the same time, the IFO fraction, in synergy with superoxide dismutase and magnesium, promotes the inhibition of lipid peroxidation of cell membranes (membrane stabilizing effect) [4, 6–8, 10].
A number of clinical studies have shown that the use of the drug Actovegin® has a positive effect on cognitive functions in cardiovascular encephalopathies, improves psychological and behavioral reactions, and is most effective in mild and moderate cognitive impairment [3, 6].
Thanks to the development and introduction into clinical practice of new medical technologies, in particular positron emission tomography (PET), there is now a real possibility of quantitative assessment of myocardial perfusion in vivo, oxygen uptake, glucose utilization, FA, and contractility. PET allows a non-invasive way to study oxygen uptake, glucose and FA metabolism with the calculation of quantitative parameters in absolute values. [18F]-2-fluoro-2-deoxyD-glucose ([18F]FDG), a glucose analogue labeled with 18-fluorine, which is not metabolized and remains unchanged in the cell cytosol, is used as a natural marker of glucose uptake and metabolism in PET. . To quantify the metabolism of FFAs in the human myocardium, [18F]-thia-hepta-decanoic acid ([18F]TDA), a long-chain false FA labeled with 18-fluorine, the accumulation of which indicates β-oxidation of FFAs, is currently used as a natural marker. in the myocardium as the main source of energy in the myocardium. To assess oxidative metabolism in PET, a model was developed using [1-11C]-acetate as a marker of myocardial oxygen uptake [1, 4].
In Fig. Figure 5 shows examples of quantitative assessment of O2 utilization of glucose metabolism (Fig. 5A) and free fatty acids (Fig. 5B) before and after intravenous infusion of 1000 mg Actovegin. Actovegin promotes a 3-fold increase in O2 utilization with a simultaneous 6-7-fold increase in glucose uptake and a similar decrease in FFA metabolism. Such dynamics of oxidative metabolism indicate Actovegin’s stimulation of aerobic oxidation, the most beneficial source of macroenergy phosphates [4].
There is evidence that the effect of Actovegin on patients with coronary artery disease complicated by acute coronary insufficiency is multicomponent, in addition to improving myocardial metabolism, it has a positive effect on the rheological properties of blood: it reduces the aggregation activity of platelets, increases the mobility of erythrocytes, and reduces blood viscosity (through a hypoglycemic effect). At the same time, Actovegin promotes angiogenesis – the development of collateral circulation [9].
According to a number of authors, the use of Actovegin by patients on the first day of development helps restore the contractile function of the myocardium of the left ventricle of MI through improving the metabolism of cardiomyocytes, eliminates electrical heterogeneity, which overall manifests itself in a reduction in the frequency of complications and early hospital mortality [4, 5].
In our studies, the use of Actovegin (from 800 to 1200 mg intravenously) in the acute phase of MI in 49 patients against the background of thrombolysis and standard therapy contributed to a more effective prevention of the development of “reperfusion” syndrome (progression of pain syndrome, increase in episodes of ventricular arrhythmias, spread of the MI zone, increase in HF ). The comparison group consisted of 67 patients with AMI who underwent artificial thrombolysis without prophylactic intravenous administration of Actovegin. Analyzes of the clinical status and studies of the pumping activity of the heart before and after treatment by group are presented in the table.
As can be seen from the table, the reduction in the incidence of reperfusion syndrome to 18.4% in the study group of AMI patients compared to the control group of AMI patients (67 AMI patients) - 34%, was mediated by improving effective diastole (elimination of “contracture” of ischemic myocardium) . All parameters of blood flow through the mitral valve, characterizing the diastolic relaxation of the left ventricular myocardium, improved statistically significantly, which contributed to an effective increase in the ejection fraction of the left ventricle - an integral indicator of the pumping activity of the heart. EF in the study group during the administration of Actovegin increased statistically significantly (p < 0.05) by 8.4% (from 39.2 ± 5.5% to 46.6 ± 2.1%) compared with the control group of patients with AMI (from 39.1 ± 2.9% to 42.7 ± 3.1%).
More effective restoration of the metabolism of ischemic myocardium during the administration of Actovegin helps to minimize electrical heterogeneity, which is clinically manifested by a decrease in the incidence of ventricular arrhythmias: in the study group after treatment they occurred in 22.4% of cases, in the comparison group - in 32.8 %.
Similar results were obtained in other clinical observations [5].
Actovegin, as an antihypoxant and secondary antioxidant, when used in clinical practice, activates aerobic respiration of cells in a state of ischemia and metabolic failure, and has a systemic effect on the body (Fig. 6). The main pharmacological actions of Actovegin are increasing the efficiency of oxygen absorption and activating glucose transport, in particular in the cardiomyocyte. Activation of aerobic oxidation processes increases the energy potential of the myocardial cell. The listed effects of Actovegin are most pronounced in the hypoxic status of the heart muscle.
Thus, the development of cardiovascular disorders during ischemic episodes is accompanied by a set of pathophysiological events, the elimination of which requires an integrated pharmacological approach, rather than a simplified unidirectional effect. Multiple cardio- and neurotropic effects suggest a simultaneous modulating effect on various damaging pathological mechanisms (inflammation, apoptosis, oxidative stress, and many others).
As a biological agent with pleiotropic effects, Actovegin in its mechanisms of action (antioxidant, antihypoxic) corresponds to the concept of an integrative therapeutic approach.
The presented review and analysis of our own experience shows that Actovegin takes an active part in restoring the balance of cellular metabolism by correcting a number of pathophysiological processes that occur during the development of IHD. Actovegin has a cardioprotective effect on the cardiovascular functional block due to its anti-apoptotic and antioxidant effects, activates the mechanisms of glucose and oxygen utilization with the normalization of intracellular energy balance, which helps improve the pumping activity of the heart.
Features of the treatment of coronary heart disease
To relieve attacks of angina, organic nitrates are most often used: nitroglycerin, isosorbide dinitrate, as well as the calcium antagonist nifedipine and the vasodilator drug validol - sublingually (under the tongue). With this route of administration, the effect occurs very quickly - within 1-3 minutes.
To prevent angina attacks, beta-blockers, calcium antagonists, ivabradine, isosorbide mononitrate, isosorbide dinitrate, pentaerythrityl tetranitrate, molsidomine, as well as cardioprotective agents (trimetazidine, meldonium) are used orally.
β-blockers and calcium antagonists additionally reduce blood pressure (antihypertensive effect) and normalize heart rhythm (antiarrhythmic effect), and are therefore indicated for the treatment of angina pectoris in patients with hypertension and/or arrhythmia.
Amiodarone has a pronounced antiarrhythmic effect, so it is used for angina pectoris against the background of heart rhythm disturbances - arrhythmias.
Tolerance (addiction) develops to organic nitrate preparations - a decrease in their effect with prolonged use. To avoid tolerance when taking organic nitrates, you need to take breaks of several days or at least overnight. In case of addiction development, preference should be given to a drug with a nitrate-like effect - molsidomine.
Antihypoxants and antioxidants in cardiological practice
TO
The key role of cardiac artery thrombosis in the formation of acute coronary syndrome, up to the development of acute myocardial infarction (AMI), is currently postulated. To replace the traditionally established conservative treatment of coronary pathology, aimed at preventing complications: dangerous rhythm disturbances, acute heart failure (AHF), limiting the area of myocardial damage (by increasing collateral blood flow), radical treatment methods have been introduced into clinical practice - recanalization of the branches of the coronary arteries by pharmacological effects (thrombolytic agents), and invasive intervention - percutaneous transluminal balloon or laser angioplasty with or without the installation of a stent(s).
Accumulated clinical and experimental experience indicates that restoration of coronary blood flow is a “double-edged sword”, i.e. in 30% or more, “reperfusion syndrome” develops, manifesting itself as additional damage to the myocardium, due to the inability of the cardiomyocyte energy system to utilize the “surging” supply of oxygen. As a result, the formation of free radical, reactive oxygen species (AA) increases, contributing to damage to membrane lipids - lipid peroxidation (LPO), additional damage to functionally important proteins, in particular, the cytochrome respiratory chain and myoglobin, nucleic acids and other structures of cardiomyocytes [ 1,7,11]. This is a simplified model of the post-perfusion metabolic circle of development and progression of ischemic myocardial damage. In this regard, pharmacological drugs for anti-ischemic (antihypoxants) and antioxidant (antioxidants) myocardial protection have now been developed and are being actively introduced into clinical practice [4,8,10,12,13].
Antihypoxants
– drugs that help improve the body’s utilization of oxygen and reduce the need for it in organs and tissues, overall increasing resistance to hypoxia. Currently, the antihypoxic and antioxidant role of Actovegin (Nycomed) has been most studied in clinical practice for the treatment of various urgent conditions of the cardiovascular system.
Actovegin
– highly purified hemodialysate obtained by ultrafiltration from the blood of calves, containing amino acids, oligopeptides, nucleosides, intermediate products of carbohydrate and fat metabolism (oligosaccharides, glycolipids), electrolytes (Mg, Na, Ca, P, K), trace elements (Si, Cu).
The basis of the pharmacological action of Actovegin is the improvement of transport, glucose utilization and oxygen absorption:
– the exchange of high-energy phosphates (ATP) increases;
– oxidative phosphorylation enzymes are activated (pyruvate and succinate dehydrogenases, cytochrome C oxidase);
– the activity of alkaline phosphatase increases, the synthesis of carbohydrates and proteins accelerates;
– the influx of K+ ions into the cell increases, which is accompanied by activation of potassium-dependent enzymes (catalases, sucrases, glucosidases);
– the breakdown of anaerobic glycolysis products (lactate, b-hydroxybutyrate) is accelerated.
The active components that make up Actovegin have an insulin-like effect. Actovegin oligosaccharides activate the transport of glucose into the cell, bypassing insulin receptors. At the same time, Actovegin modulates the activity of intracellular glucose carriers, which is accompanied by an intensification of lipolysis. What is extremely important is that Actovegin’s action is insulin-independent and persists in patients with insulin-dependent diabetes mellitus, helps slow the progression of diabetic angiopathy and restore the capillary network due to new vascular formation [2,9].
The improvement in microcirculation, which is observed under the influence of Actovegin, is apparently associated with an improvement in the aerobic metabolism of the vascular endothelium, which promotes the release of prostacyclin and nitric oxide (biological vasodilators). Vasodilation and decreased peripheral vascular resistance are secondary to activation of oxygen metabolism in the vascular wall.
Thus, the antihypoxic effect of Actovegin is summarized through improved glucose utilization, oxygen absorption and a decrease in myocardial oxygen consumption as a result of a decrease in peripheral resistance.
The antioxidant effect of Actovegin is due to the presence in this drug of high superoxide dismutase activity, confirmed by atomic emission spectrometry, the presence of magnesium preparations and microelements included in the prosthetic group of superoxide dismutase. Magnesium is an obligatory participant in the synthesis of cellular peptides; it is part of 13 metalloproteins, more than 300 enzymes, including glutathione synthetase, which converts glutamate into glutamine [9].
The accumulated clinical experience of intensive care units allows us to recommend the administration of high doses of Actovegin: from 800–1200 mg to 2–4 g. Intravenous administration of Actovegin is advisable:
– for the prevention of reperfusion syndrome in patients with AMI, after thrombolytic therapy or balloon angioplasty;
– patients during the treatment of various types of shock;
– patients suffering from circulatory arrest and asphyxia;
– patients with severe heart failure;
– patients with metabolic syndrome X.
Antioxidants
– block the activation of free radical processes (AA formation) and lipid peroxidation (LPO) of cell membranes that occur during the development of AMI, ischemic and hemorrhagic strokes, acute disorders of regional and general circulation. Their action is realized through the reduction of free radicals into a stable molecular form that is not able to participate in the autoxidation chain. Antioxidants either directly bind free radicals (direct antioxidants) or stimulate the tissue antioxidant system (indirect antioxidants).
Energostim
– a combined preparation containing nicotinamide adenine dinucleotide (NAD), cytochrome C and inosine in the ratio: 0.5, 10 and 80 mg, respectively.
In AMI, disturbances in the energy supply system occur as a result of the loss by cardiomyocytes of NAD, a coenzyme of dehydrogenase of glycolysis and the Krebs cycle, and cytochrome C, an enzyme of the electron transport chain, which is associated with ATP synthesis in mitochondria (Mx) through oxidative phosphorylation. In turn, the release of cytochrome C from Mx leads not only to the development of energy deficiency, but also promotes the formation of free radicals and the progression of oxidative stress, ending in cell death through the mechanism of apoptosis. After intravenous administration, exogenous NAD, penetrating through the sarcolemma and membranes of Mx, eliminates the deficiency of cytosolic NAD, restores the activity of NAD-dependent dehydrogenases involved in the synthesis of ATP by the glycolytic route, and promotes the intensification of the transport of cytosolic proton and electrons in the respiratory chain of Mx. In turn, exogenous cytochrome C in Mx normalizes the transfer of electrons and protons to cytochrome oxidase, which overall stimulates the ATP-synthesizing function of oxidative phosphorylation of Mx. However, eliminating the deficiency of NAD and cytochrome C does not completely normalize the “conveyor” of ATP synthesis in the cardiomyocyte, since it does not have a significant effect on the content of individual components of adenyl nucleotides involved in the respiratory chain of cells. Restoration of the total content of adenyl nucleotides occurs with the introduction of inosine, a metabolite that stimulates the synthesis of adenyl nucleotides. At the same time, inosine enhances coronary blood flow, promotes the delivery and utilization of oxygen in the microcirculation area.
Thus, it is advisable to combine administration of NAD, cytochrome C and inosine
for effective impact on metabolic processes in cardiomyocytes subjected to ischemic stress.
Energostim, according to the mechanism of pharmacological effects on cellular metabolism, has a combined effect on organs and tissues: antioxidant and antihypoxic. Due to the composite composition, Energostim, according to various authors, is many times more effective in treating MI as part of traditional treatment than other world-recognized antihypoxants: lithium hydroxybutyrate, riboxin (inosine) and amitazole by 2–2.5 times, and amitazole by 3–2.5 times. 4 times – carnitine (mildronate), piracetam, olifen and solcoseryl, 5–6 times – cytochrome C, aspisol, ubiquinone and trimetazidine [1,11]. Recommended doses of Energostim in the complex therapy of MI: 110 mg (1 bottle) in 100 ml of 5% glucose 2-3 times a day for 4-5 days. All of the above allows us to consider Energostim a drug of choice in the complex therapy of MI, for the prevention of complications resulting from metabolic disorders in cardiomyocytes [1,3].
Coenzyme Q10
- a vitamin-like substance, was first isolated in 1957 from bovine heart mitochondria by the American scientist F. Crane. K. Folkers in 1958 determined its structure. The second official name for CoQ10 is ubiquinone (the ubiquitous quinone), as it is found in varying concentrations in virtually all animal tissues. In the 60s, the role of Q10 as an electron carrier in the Mx respiratory chain was demonstrated. In 1978, P. Mitchell proposed a scheme explaining the participation of coenzyme Q10 both in electron transport in mitochondria and in the coupling of the processes of electron transport and oxidative phosphorylation, for which he received the Nobel Prize [8].
Coenzyme Q10 effectively protects the lipids of biological membranes and lipoprotein blood particles (phospholipids - “membrane glue”) from the destructive processes of peroxidation, protects the body’s DNA and proteins from oxidative modification as a result of the accumulation of reactive oxygen species (AA). Coenzyme Q10 is synthesized in the body from the amino acid tyrosine with the participation of B vitamins C, folic and pantothenic acids, and a number of microelements. With age, the biosynthesis of coenzyme Q10 progressively decreases, and its consumption during physical and emotional stress, in the pathogenesis of various diseases and oxidative stress increases [5].
More than 20 years of experience in clinical studies of the use of coenzyme Q10 in thousands of patients convincingly prove the role of its deficiency in the pathology of the cardiovascular system, which is not surprising, since it is in the cells of the heart muscle that the energy needs are greatest. The protective role of coenzyme Q10 is due to its participation in the processes of energy metabolism of the cardiomyocyte and its antioxidant properties. The uniqueness of the drug under discussion lies in its regenerative ability under the influence of the body’s enzyme systems. This distinguishes coenzyme Q10 from other antioxidants, which, while performing their function, are irreversibly oxidized themselves, requiring additional administration [6].
The first positive clinical experience in cardiology with the use of coenzyme Q10 was obtained in the treatment of patients with dilated cardiomyopathy and mitral valve prolapse: convincing data were obtained in improving myocardial diastolic function. The diastolic function of a cardiomyocyte is an energy-intensive process and, under various pathological conditions, the cardiovascular system consumes up to 50% or more of all the energy contained in ATP synthesized in the cell, which determines its strong dependence on the level of coenzyme Q10.
Clinical studies of recent decades have shown the therapeutic effectiveness of coenzyme Q10 in the complex treatment of coronary artery disease
, arterial hypertension, atherosclerosis and chronic fatigue syndrome. The accumulated clinical experience allows us to recommend the use of Q10 not only as an effective drug in the complex treatment of CV diseases, but also as a means of preventing them.
The prophylactic dose of Q10 for adults is 15 mg/day, therapeutic doses are 30–150 mg/day, and in cases of intensive care – up to 300–500 mg/day. It should be taken into account that high therapeutic doses of oral coenzyme Q10 are associated with difficulty in absorbing fat-soluble substances, therefore, a water-soluble form of ubiquinone has now been created to improve bioavailability.
Experimental studies have shown the preventive and therapeutic effect of coenzyme Q10 in reperfusion syndrome, documented by the preservation of the subcellular structures of cardiomyocytes subjected to ischemic stress and the function of oxidative phosphorylation of Mx [5,6].
Clinical experience with the use of coenzyme Q10 is so far limited to the treatment of children with chronic tachyarrhythmias, long QT syndrome, cardiomyopathies, and sick sinus syndrome [14].
Thus, a clear understanding of the pathophysiological mechanisms of damage to cells of tissues and organs subjected to ischemic stress, which are based on metabolic disorders - lipid peroxidation, occurring in various CV diseases, dictate the need to include antioxidants and antihypoxants in the complex therapy of urgent conditions.
Literature:
1. Andriadze N.A., Sukoyan G.V., Otarishvili N.O, et al. Direct-acting antihypoxant Energostim in the treatment of AMI. Ross. Honey. Vesti, 2001, No. 2, 31–42.
2. Boyarinov A.P., Penknovich A.A., Mukhina N.V. Metabolic effects of the neurotropic action of actovegin under hypoxic conditions. Actovegin. New aspects of clinical application. M., 2002, 10–14.
3. Dzhanashiya P.Kh., Protsenko E.A., Sorokoletov S.M. Energostim in the treatment of chronic forms of ischemic heart disease. Ross. Card. Zh., 1988, No. 5, 14–19.
4. Zakirova A.N. Correlations of lipid peroxidation, antioxidant protection and microrheological disorders in the development of ischemic heart disease. Ter.archive, 1966, No. 3, 37–40.
5. Kapelko V.I., Ruuge E.K. Study of the effect of coenzyme Q10 (ubiquinone) in cardiac ischemia and reperfusion. The use of the antioxidant drug kudesan (coenzyme Q 10 with vitamin E) in cardiology. M., 2002. 8–14.
6. Kapelko V.I., Ruuge E.K. Research on the effect of Kudesan on stress-induced damage to the heart muscle. The use of the antioxidant drug kudesan (coenzyme Q10 with vitamin E) in cardiology. M., 2002, 15–22.
7. Kogan A.Kh., Kudrin A.N., Kaktursky L.V. and others. Free radical peroxide mechanisms of the pathogenesis of ischemia and MI and their pharmacological regulation. Pathophysiology, 1992, No. 2, 5–15.
8. Korovina N.A., Ruuge E.K. The use of coenzyme Q10 in prevention and treatment. The use of the antioxidant drug kudesan (coenzyme Q10 with vitamin E) in cardiology. M., 2002, 3–7.
9. Nordvik B. Mechanism of action and clinical use of the drug Actovegin. Actovegin. New aspects of clinical application. M., 2002, 18–24.
10. Rumyantseva S.A. Pharmacological characteristics and mechanism of action of Actovegin. Actovegin. New aspects of clinical application. M., 2002, 3–9.
11. Slepneva L.V. Alekseeva N.I., Krivtsova I.M. Acute organ ischemia and early post-ischemic disorders. M., 1978, 468–469.
12. Smirnov A.V., Krivoruchka B.I. Antihypoxants in emergency medicine. Anest. I resuscitation, 1998, No. 2, 50–57.
13. Shabalin A.V., Nikitin Yu.P. Cardiomyocyte protection. Current status and prospects. Cardiology, 1999, No. 3, 4–10.
14. Shkolnikova M.A. Report of the Association of Pediatric Cardiologists of Russia on the use of Kudesan. The use of the antioxidant drug kudesan (coenzyme Q10 with vitamin E) in cardiology. M., 2002, 23.